-
About Bladder Cancer
Smoking and industrial chemicals raise your risk. Get more facts and connect to in-depth info.
-
Upper Tract Urothelial Cancer
At the GBCI, our specialized team is developing new therapies and approaches to UTUC.
-
Bladder Cancer Treatment
From proven protocols to promising clinical trials, you have options. We'll educate and guide you.
-
Our Approach to Care
A multidisciplinary team collaborates to provide customized precision care every step of the way.
-
Grand Rounds Educational Series
View recordings of past webinars or register for upcoming sessions.
-
Women & Bladder Cancer
Learn more about our Women's Bladder Cancer Program, or register for our upcoming women-run support groups.
In-person clinical appointments
The Greenberg Bladder Cancer Institute offers in-person clinical appointments for newly diagnosed bladder cancer, bladder cancer which has recurred (sometimes referred to as metastasized) and upper tract urothelial cancer (commonly referred to as UTUC). We have specialized clinical programs to serve patients and families at all stages of bladder cancer. We are unable to do telemedicine consults or informally comment on a treatment plan. To start the process of seeing our bladder cancer team or our upper tract urothelial team, please send us an email to [email protected] or [email protected]
As a virtual institute, we partner with other leading experts around the world to share knowledge and collaborate on a global research agenda. Exciting advancements in bladder cancer research are happening right now. At the Greenberg Institute, we’re working to ensure these developments make a difference in patient lives — the sooner the better.
ON THIS PAGE:
OUR APPROACH CLINICAL TRIALS ABOUT THE INSTITUTE RESEARCH HIGHLIGHTS RESOURCES PATIENT STORIES
Introduction: Johns Hopkins Greenberg Bladder Cancer Institute
The Johns Hopkins Greenberg Bladder Cancer Institute (GBCI) is making a significant impact on bladder cancer treatment, focusing on developing a cure through research. Our multidisciplinary, collaborative clinical approach utilizes innovative strategies - including the genetic sequencing of individual tumors - to provide more treatment choices to patients and help improve outcomes. Advances at the GBCI are having a ripple effect on bladder cancer treatment advances worldwide.
Regular Team Meetings Prevent Care Fragmentation
Team members meet together in the clinic — weekly, though sometimes daily — to review your case: test results, treatment plans, progress, next steps. We believe that collaborative care will help you make the right informed decisions. This team approach allows for all of those caring for you across disciplines can offer their best informed opinion in a one-stop meeting to best review and support the best care options for you moving forward.
You can feel confident knowing that your care team is in sync.
Multi-Disciplinary Clinics Add Convenience
The unique feature of our bladder cancer Multi-Disciplinary program is that patients have the opportunity to be in the same room as the many members of their care team. Our surgical and medical oncologists hold bladder cancer multi-disciplinary clinics on the same day at the same place, and even sit together to review options.
You can consult with both doctors when necessary, and care teams can streamline the scheduling, follow-up and research involved in each treatment plan.
Access to Cutting-Edge Treatments and Techniques and Clinical Trials
As a global hub for advancing the understanding and treatment of bladder cancer, the Greenberg Institute is a leader in the latest research developments. We are eager to share that knowledge.
Our clinicians aren't just dedicated to patient care — they're renowned bladder cancer researchers actively working to develop new and better ways to fight the disease. With insight into newly available and on-the-horizon treatments, our team can offer suggestions on the suitability of clinical trial opportunities that may hold promise for many patients.
Tumor Sequencing for Greater Precision
Because bladder cancer treatment can be invasive and have a lifelong impact, it's vital that individual treatment plans are customized to minimize side effects and maximize outcomes. Because cancer biology dictates clinical behavior and response or resistance to treatment, we need to have a deep understanding of cancer biology to make informed treatment decisions. That's why at Johns Hopkins, our multidisciplinary approach includes tumor sequencing for every patient. In patients with advanced stage disease that has spread beyond the bladder, tumor sequencing results are a component of standard medical care and their results will be shared and discussed with each patient to collectively decide on optimal therapy approaches. In early stage patients with disease confined to the bladder, the use of tumor sequencing results to guide therapy has not yet been established as part of standard care. In such patients, we plan to perform tumor sequencing as part of an ongoing GBCI research effort to identify and understand the key genetic targets responsible for tumor growth, invasion, and spread. As any results of these research efforts will be preliminary, research tumor sequencing results will be analyzed in a manner in which individual patient identifying information is removed prior to sequencing analyses. As such, these de-identified tissue sequencing results will not be communicated back to individual patients.
By comparing the tumor's gene expression patterns and mutations to large public databases of bladder cancer tumors, we can identify the exact cancer type and make predictions about whether certain protocols, like neoadjuvant chemotherapy or radical cystectomy, will ultimately improve outcomes. This unique form of "precision medicine" helps our team assess risk and plan for the most efficient and effective treatments.
Continuity Throughout the Course of Care
Your journey should be as seamless as possible, from diagnosis through treatment and beyond. Consistency and communication are key to reducing unnecessary stress and helping patients heal.
Our oncology-trained nurse practitioner works closely with the multidisciplinary care team to guide you through every step of the process. Patients receiving intravesical chemotherapy or immunotherapy have a dedicated nurse who will administer treatment at every visit. Patients with stomas or neobladders are managed by a dedicated stoma nurse before surgery, during the hospital stay and after discharge.
Clinical Trials
The Promise of Research
No two bladder cancer cases are alike, which is why the Johns Hopkins Greenberg Bladder Cancer Institute team takes an individualized approach with each patient. Our investigators are committed to improving the lives of bladder cancer patients and believe that clinical trials are critical to realizing that goal. Within the Greenberg Institute, clinical trials are presented as an initial option to all patients. If our multidisciplinary team determines that a clinical trial option is not available for a particular patient, standard of care options always remain a therapy option.
Clinical trials provide patients with access to new bladder cancer treatment approaches in their early stages of discovery. These may include new immunotherapy drugs or surgical techniques, novel protocols for specific cancer stages, or trials to determine the effectiveness of different treatment combinations.
See FAQs about clinical trials.
How to Enroll
Many of the studies available at Johns Hopkins are being directed by physicians on the Johns Hopkins Greenberg Institute team, which is dedicated to advancing bladder cancer research. Our team is very knowledgeable about trials and collaborates with many other clinical trial enrollments.
Our multi-disciplinary team of experts will discuss the pros and cons of a particular trial and whether you meet eligibility requirements. They will be with you from inception and through the clinical trial journey.
Find a Clinical Trial
View current trials offered at the Johns Hopkins Kimmel Cancer Center for bladder cancer patients.
About the Institute
The Johns Hopkins Greenberg Bladder Cancer Institute, based in Baltimore, Maryland, is the first of its kind that is dedicated to improving the lives and outcomes of patients with bladder cancer and their families. In May 2014, the Institute was established with a $15 million gift from Baltimore-area commercial real estate developer Erwin L. Greenberg and his wife, Stephanie Cooper Greenberg — part of a $45 million co-investment with The Johns Hopkins University.
Read more about our goals in the Greenberg Bladder Cancer Institute Case Statement.
-
Our Mission
We want to be a conduit for collaboration for all who are dedicated to fighting bladder cancer.
-
The Greenbergs
Their generosity could help save thousands of lives. Read about the Greenbergs and their founding gift.
-
Make a Gift
We're making progress. Can you help us get there faster? See what your gift can do and donate today.
The Institute was inspired by the tens of thousands of men and women diagnosed with bladder cancer each year. For 50 years, there has been little new to offer patients, but that is changing. As part of the revolution in bladder cancer research and clinical discovery, our team is bringing gene therapy, immunotherapy, targeted therapies, tumor sequencing, clinical trials, drug development and other innovations, all for the benefit of bladder cancer patients.
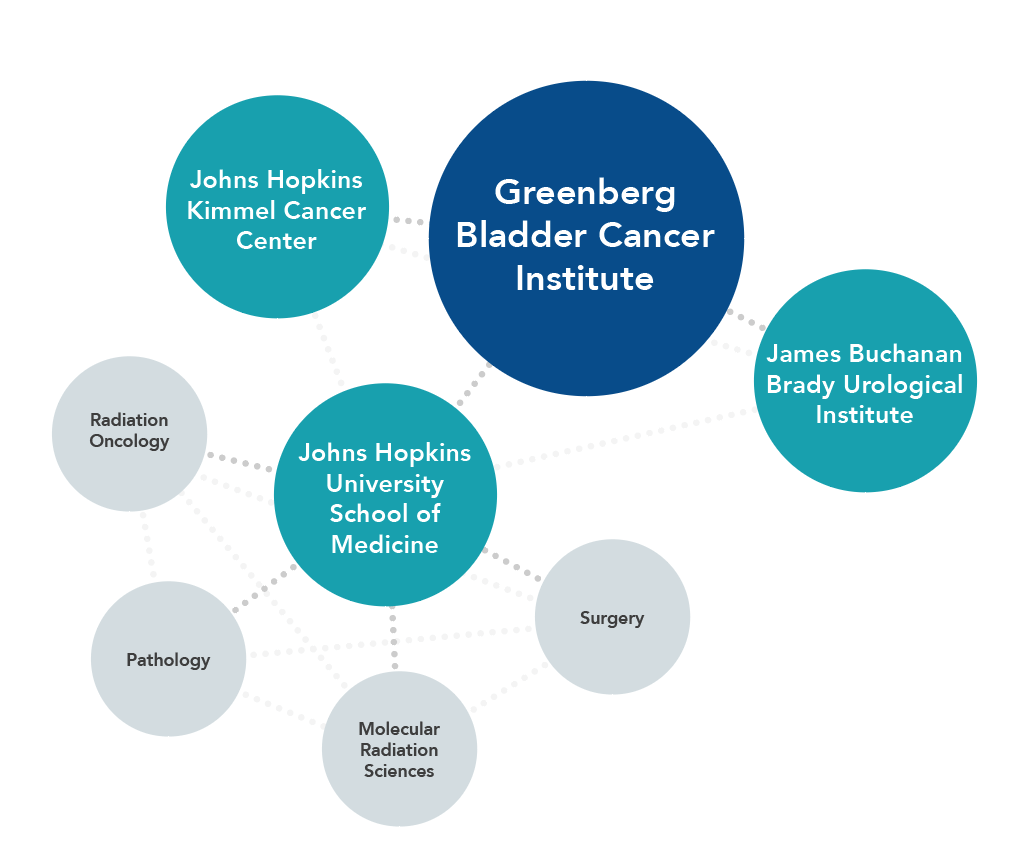
We strive to be a collaborative virtual network that draws upon the world’s best expertise — from Johns Hopkins and beyond — to further the scientific understanding of bladder cancer and develop new methods for preventing, treating and curing the disease. Our multidisciplinary research teams include experts from the Johns Hopkins Kimmel Cancer Center and James Buchanan Brady Urological Institute, as well as faculty members from the Johns Hopkins University School of Medicine’s departments of Radiation Oncology and Molecular Radiation Sciences, Pathology, and Surgery.
Our Mission
Long considered the “invisible cancer,†the eradication of bladder cancer is our sole mission and purpose. We are dedicated to better understanding bladder cancer and improving its treatment.
As the forerunner in this global collaborative network, the Institute is organizing group moonshot-level research efforts, with the goal of producing positive clinical impact in the near term and for the future.
As a grant-making institution, the Institute encourages investigators to take on innovative bladder cancer research projects that advance science and clinical practice.
As a cutting-edge clinical provider, the Institute provides each patient with individualized, science-based multidisciplinary care team that considers every available option and utilizes the latest treatment techniques and clinical trials.
Bladder Cancer Q&A with urologist Max Kates, M.D.
Urologist Max Kates, M.D., answers common questions about bladder cancer, including who is at risk, signs and symptoms, as well as the latest treatment options at the Johns Hopkins Greenberg Bladder Cancer Institute.
Research Highlights

Stay Up to Date with Discovery
Researchers at the Johns Hopkins Greenberg Bladder Cancer Institute, in collaboration with other leading institutions, are developing and evaluating innovative approaches to the way the world diagnoses, treats and prevents bladder cancer.
After decades of lagging behind other diseases in the pace of funding and discovery, bladder cancer is finally getting the attention and research funding it deserves. The technology and science have caught up to developments that have been made for other diseases, and targets are showing encouraging results specific to bladder cancer.
We’re making progress every day, and some of our work is already having a positive impact on patients involved with clinical trials.
Get the highlights on current studies and recent developments in bladder cancer research:
- Immunotherapy
- Targeted therapies
- Bladder preservation
- Genetics and epigenetics
- Urinary reconstruction
Learn more about the JHGBCI in our e-newsletter.

Issue 5, Winter/Spring 2022
 Issue 4, Spring 2021
Issue 4, Spring 2021
DISCOVERY: a publication of the Patrick C. Walsh Prostate Cancer Research Fund, the Brady Urological Institute, and Johns Hopkins Medicine
- *NEW* DISCOVERY Volume XIX, Winter 2023 *NEW*
- DISCOVERY Volume XVIII, Winter 2022
- DISCOVERY Volume XVII, Winter 2021
- DISCOVERY Volume XVI, Winter 2020
- DISCOVERY Volume XV, Winter 2019
- DISCOVERY Volume XIV, Winter 2018
- DISCOVERY Volume XIII, Winter 2017
- DISCOVERY Volume XII, Winter 2016
- DISCOVERY, Volume XI, Winter 2015
- Prior editions of DISCOVERY
View bladder cancer clinical trials at Johns Hopkins.
Read about the exciting research projects we’re currently funding.
Research Updates
- Newly Discovered Mutation on HOXB13 is Linked to More Aggressive Cancer in Black Men (Discovery, 11/14/2022)
- High-Risk Non Muscle-Invasive BCG Unresponsive Bladder Cancer - Molecular Subtypes - Woonyoung Choi (Uro Today, 10/14/2020)
- Sexual Dysfunction, Molecular Markers - Johns Hopkins Greenberg Bladder Cancer Institute Awards Aim to Learn How Bladder Cancer Affects Women (01/31/2019)
- Dr Bill Nelson talks with Dr Jean Hoffman-Censits and Dr Armine Smith about the complications that arise in diagnosing and treating bladder cancer in women (11/15/2018)
- 2018 Peer Reviewed Cancer Highlight - IN FOCUS: BLADDER CANCER (Congressionally Directed Medical Reseach Programs, 10/10/2018)
- Dr. Woonyoung Choi awarded Best Basic Science ePoster at the 38th Congress of the SIU in Seoul, South Korea (MP02.06, page 38, 10/7/2018)
- Dr. David McConkey discusses new paradigms in the treatment of bladder cancer with Dr. Bill Nelson (07/26/2018)
- Developments in the Management of BCG-Unresponsive NMIBC (Renal and Urology News, 5/17/2018)
- FDA Breakthrough Therapy Designation Announced for Enfortumab Vedotin in Locally Advanced or Metastatic Urothelial Cancer (03/26/2018)
- Gene-based test for urine detects, monitors bladder cancer (03/22/2018)
- New research discovers two proteins pivotal to maintaining cancer stem cells, which cause chemotherapy resistance (01/24/2018)
- Johns Hopkins Scientists Develop Experimental "Nano-Chemo" Particle to Treat Bladder Cancer (10/12/2017)
- The State of the Bladder Cancer Battle (05/10/2017)
- Hope for the Future: an Engineered Bladder (02/15/2017)
- Dr. David J. McConkey to Discuss Immunology in Bladder Cancer During Keynote Lecture (02/09/2017)
- Johns Hopkins scientists zero in on how cancers resist immunotherapy treatment (01/06/2017)
- The Future of Bladder Cancer Research (02/15/2016)
Immunotherapy
For years, physicians have tried to stimulate the immune system through vaccines or in bladder cancer patients using a bacteria-derived drug called BCG. More recently, scientists have discovered that immune cells express molecules called checkpoints, or brake molecules. Blocking different types of checkpoints with a kind of immunotherapy called an immune checkpoint blockade unleashes the immune system’s natural potential to fight cancer.
On the Horizon: Improved Immunotherapy for Bladder Cancer
Recent breakthrough clinical trials at a few of our partner institutions have resulted in the first new drug approved for metastatic bladder cancer in two decades and evidence that combinations of existing immune checkpoint blockades may be effective in bladder cancer. These discoveries — led by Jonathan Rosenberg, M.D., of Memorial Sloan Kettering Cancer Center; Elizabeth Plimack, M.D., of Fox Chase Cancer Center; and Padmanee Sharma, M.D, Ph.D., of the MD Anderson Cancer Center — have excited the field and set the stage for additional research.
Researchers at the Johns Hopkins Greenberg Bladder Cancer Institute are focused on working with the Bloomberg~Kimmel Institute for Cancer Immunotherapy to investigate better combinations of immunotherapies that could achieve a higher response rate for more patients and better methods to predict which bladder cancer patients are likeliest to benefit from these therapies.
Targeted Therapies
The discovery that muscle-invasive bladder cancer can be grouped into at least four molecular subtypes has resulted in many research studies that seek to develop more targeted therapies and approaches, particularly around determining which patients are good candidates for chemotherapy.
Systemic (Drug) Therapy
Our medical oncology experts are studying biologic therapies that block cancer growth in patients who have specific genetic mutations in their cancers. Dovitinib binds to and inhibits a particular mutation, which may hinder tumor cell proliferation and induce tumor cell death.
Oncologist Noah Hahn, M.D., discusses what’s new in bladder cancer research.
Award-Winning Study
David McConkey, Ph.D., the director of the Greenberg Bladder Cancer Institute, won the 2016 Bladder Cancer Advocacy Network’s Innovation Award for his project “Targeting FGFR3 in Muscle-Invasive Bladder Cancer.” The project is a collaboration between Johns Hopkins and Arlene Siefker-Radtke, M.D., of the MD Anderson Cancer Center. It will investigate strategies for bladder cancer that is resistant to standard therapies. Learn more about the award and McConkey’s study.
Bladder Preservation
Radical cystectomy is the standard treatment for muscle-invasive bladder cancer, but because this is an invasive procedure, many researchers are investigating ways to treat the disease while preserving the bladder.
A subset of patients at the Greenberg Bladder Cancer Institute may already benefit from bladder preservation, thanks to a program led by Theodore DeWeese, M.D., director of the Department of Radiation Oncology and Molecular Radiation Sciences. He identifies muscle-invasive bladder cancer patients whose individual cases may respond to trimodality therapy alone, which includes tumor resection, chemotherapy and radiation.
Urologic oncologist Noah Hahn, M.D., is working with other leaders in the bladder cancer field to investigate a treatment approach that could allow more muscle-invasive bladder cancer patients to keep their bladders. The concept also incorporates immunotherapy drugs called checkpoint inhibitors, which have been shown to be effective on some types of tumors.
Genetics and Epigenetics
Genetic Biomarkers
Johns Hopkins researchers are on the hunt for bladder cancer biomarkers that appear in urine. Such markers could potentially serve as noninvasive, DNA-based tests for early diagnosis of bladder cancer in patients at high risk for developing the disease or early recurrence among bladder cancer patients in remission. They also have the potential to help predict who will respond to therapy.
In 2013, a team led by Bert Vogelstein, M.D., Nickolas Papadopoulos, Ph.D., Luis Diaz, M.D., and Ken Kinzler, Ph.D., from the Ludwig Cancer Research Center; Georges Netto, M.D., from the Division of Urologic Pathology; and Trinity Bivalacqua, M.D., Ph.D., from the Brady Urological Institute found a high prevalence of mutations in the promoter of a gene called telomerase reverse transcriptase (TERT) in a wide range of bladder cancer precursor lesions. As part of the study, they studied the presence of TERT promoter mutations in urine samples from 14 patients who had been treated for early-stage bladder cancer. Among patients whose tumors harbored TERT promoter mutations, the same mutations were present in follow-up urine samples from seven of eight patients whose cancer recurred. The mutations were not present in follow-up urine samples from six patients whose cancer did not recur.
The work was published in the journal Cancer Research. In 2014 and 2015, a team led by Netto received grants from the Greenberg Bladder Cancer Institute to continue their work.
BCAN Bladder Cancer Genomics Consortium Project
The Johns Hopkins Greenberg Bladder Cancer Institute is one of eight leading U.S. bladder cancer programs participating in a large-scale study launched by the Bladder Cancer Advocacy Network and its Bladder Cancer Genomics Consortium (BCGC). The study is a collaborative effort to sequence the genomic profiles of metastatic bladder cancer patients and house the information in a repository that is accessible to all partner institutions.
The data collected can be used by any partner institution or group for research into targeted genetic treatment approaches and to develop clinical trials. The consortium plans to track the results of this collaborative effort to ensure it is making a substantial, real-world impact.
Two members of the Johns Hopkins Greenberg Bladder Cancer Institute — David McConkey, Ph.D., its director, and Noah Hahn, M.D. — are on the BCGC Advisory Committee.
Urinary Reconstruction
Tissue Engineering
Researchers at the Johns Hopkins Greenberg Bladder Cancer Institute, directed by oncology and urology surgeon Trinity Bivalacqua, M.D., Ph.D., have created a novel tube to empty urine from the body. The tube, for patients who have had their bladders removed as part of treatment for bladder cancer and other conditions, is grown from each patient’s own cells.
Resources for Patients and Caregivers

Ongoing support can help patients and their families throughout the bladder cancer journey, whether the patient has been recently diagnosed, is undergoing treatment or is learning to adjust to life after cancer.
The following resources will help you get answers to your questions and connect to other people who have been affected by bladder cancer.
- Patient and Family Services at Johns Hopkins
- Find a Support Group
- Educational Resources
- Survivorship
- Advocacy
Many of these resources are provided courtesy of the Bladder Cancer Advocacy Network, a key partner of the Greenberg Bladder Cancer Institute. Based in Bethesda, Maryland, the network provides information and support to patients, while advocating for research funding and greater collaboration within the scientific community.
Patient and Family Services at Johns Hopkins
The Harry J. Duffey Family Patient and Family Services Program supports patients and families of the Johns Hopkins Kimmel Cancer Center. The program has resources to address the many needs of bladder cancer patients and caregivers:
- PracticalPlanning your visit, navigating the hospital, handling finances and arranging temporary housing
- Clinical Pain and palliative care and cancer rehabilitation
- Psychological, Social, Emotional and SpiritualManaging stress, accessing counseling services and spiritual support, and communicating with loved ones
Find an Educational and Support Group
At Johns Hopkins
- Email us at [email protected] if you would like to be added to our email distribution list for support group updates and meeting announcements.
- Living with Cancer Resource Program - Find upcoming support groups, webinars and Q&A sessions dealing with a variety of cancer-related topics.
Bladder Cancer Advocacy Network Programs
- Online Inspire Community - Ask anonymous questions and connect with other bladder cancer patients and caregivers.
- Survivor2Survivor Program - The network connects newly diagnosed patients with volunteer survivors who have had similar experiences. Call 1-888-901-2226, ext. 206.
Around the US and Canada
- Hope Connections for Cancer Support - Professionally facilitated bladder cancer group meets monthly in Bethesda, Maryland.
Educational Resources
General Information
- Bladder Cancer Basics - Signs and symptoms, diagnosis, and general treatment information from the Johns Hopkins Medicine Health Library.
- Bladder Cancer Risk Factors - The American Cancer Society provides a list of specific risk factors linked to bladder cancer.
- For the Newly Diagnosed - Bladder Cancer Advocacy Network-compiled guidance about being your own best advocate, from people who are living with bladder cancer.
- Women and Bladder Cancer - The Bladder Cancer Advocacy Network offers information unique to women and highlights several female survivors and their personal stories.
- Bladder Cancer Prognosis - The American Cancer Society provides relative survival rates for bladder cancer based on years and stage.
Videos
- Bladder Cancer Q&A - Surgeon Armine Smith, M.D., from the Johns Hopkins Brady Urological Institute answers questions about bladder cancer, diagnosis and treatment options.
- Patient Stories - Johns Hopkins bladder cancer patients share their personal stories.
- Caregiver Video Series - "Walking on Eggshells" gives practical advice to caregivers on communication, advocating for the patient, financial concerns, end-of-life issues and more.
Survivorship
- Johns Hopkins Survivorship Resources - What to expect after cancer treatment, including transitioning to primary care, maintaining the work-life-health balance, addressing insurance issues and accessing support.
- Living Well During and After Treatment - Tips from the American Cancer Society on nutrition, physical activity, work, post-treatment health care and living with the possibility of recurrence.
Advocacy
After an experience with bladder cancer, many patients and their families find it rewarding to get involved in the cause. There are many ways you can help make a difference, including volunteering, donating, raising awareness for the disease and the need for research funding, and sharing your journey with other patients and caregivers.
Patient Stories
Patients and their physicians choose Johns Hopkins for bladder cancer care because our team of urologists, oncologists, nurses, pathologists and radiologists is one of the most experienced in the world. Hear the personal stories of a few of the countless bladder cancer patients who have benefited from our individualized, innovative approach.
John's Story: Radical Cystectomy with Ileal Conduit
After being diagnosed with bladder cancer, John came to the Brady Urological Institute at Johns Hopkins for its innovative diagnostic and treatment options. When nonmuscle-invasive bladder cancer recurred as muscle-invasive, John opted for bladder removal.
Warren's Story: Partial Cystectomy for Non-Muscle Invasive Bladder Cancer
When his non-muscle invasive bladder cancer did not respond to treatment, Warren met with Noah Hahn, M.D., at the Johns Hopkins Greenberg Bladder Cancer Institute. The unique approach fostered by our multidisciplinary team was able to spare his bladder, facilitating a full recovery and a quick return to his dental practice







