-
Nirmish Singla, MD MSC

- Director of Translational Research in GU Oncology
- Associate Professor of Urology
Upper Tract Urothelial Cancer
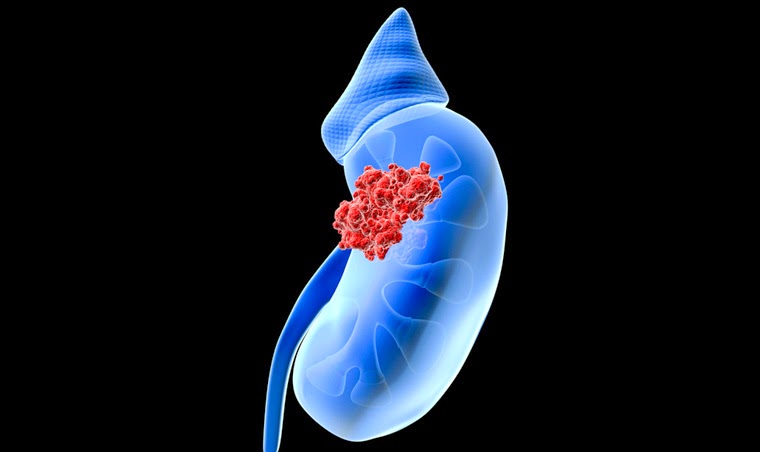
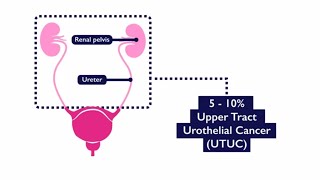
Urothelial cancer refers to a cancer of the lining of the urinary system. While the majority of urothelial cancers (approximately 90-95%) arise in the bladder, upper tract urothelial cancers (UTUCs) correspond to a subset of urothelial cancers that arise in the lining of the kidney (called the renal pelvis) or the ureter (the long, thin tube that connects that kidney to the bladder). As the lining of the bladder, kidney and ureter are the same, there are many similarities and some differences between UTUCs and bladder cancers. For instance, both bladder cancers and UTUCs can present with hematuria (blood in the urine). However, UTUCs can block the ureter or kidney, causing swelling (known as hydronephrosis) and infections, and they can even affect kidney function in some patients.
-
Reach Out Directly
The UTUC team is dedicated to providing the best possible service. Email us at [email protected] to learn more.

-
UTUC Clinical Trials
Learn more about current and ongoing UTUC clinical trials at the Greenberg Bladder Cancer Institute.
-
Enroll in our nationwide trial
Learn about our ongoing Phase III Clinical Trial for UTUC patients, currently recruiting nationwide.

Similar to bladder cancers, UTUCs can develop as low- or high-grade tumors. In general, low-grade tumors are not invasive and very rarely spread from the kidney or ureter. However, they often recur and management involves treating visible tumors and trying to preserve the urinary tract – as these tumors are more likely to recur in the urinary system than they are to spread. High-grade tumors have an aggressive appearance under a microscope and are assumed invasive in the kidney or ureter. In the bladder, a thick bladder muscle (called the detrusor) acts as a barrier to confine invasive cancers but in the kidney and ureter, this muscle does not exist. High-grade UTUC has the potential to spread from the kidney or ureter and is most often treated with surgical removal of the kidney and ureter – an operation called radical nephrouretectomy. High-grade UTUC can be aggressive and an expert may recommend systemic therapy (for example, chemotherapy) before or after surgery to reduce the risk of recurrence elsewhere in the body. An expert pathologist is often enlisted to determine low- and high-grade urothelial cancers, as this distinction can dramatically impact management choices.
Upper Tract Urothelial Cancer overview
Upper tract urothelial cancer (UTUC) is a subset of urothelial cancer that is found in the renal pelvis of the ureter. At Johns Hopkins, we have a team of specialist in the Greenberg Bladder Cancer Institute who work together to treat and manage patients with this disease.
Watch this short video to learn more about UTUCs.
Upper Tract Urothelial Cancer: It's a Team Effort
UTUC Multidisciplinary Clinic Co-Directors Drs. Jean Hoffman-Censits and Nirmish Singla discuss how doctors get together, go over cases, and share their fields of expertise in bladder and upper tract cancers. They provide a behind-the-scenes look at what takes place, who is present, and how care plans for patients are considered. Learn about the power of multidisciplinary care planning. (From the GBCI Virtual Grand Rounds series, recorded 10/12/2021)
At the Greenberg Bladder Cancer Institute, we have a specialized multidisciplinary medical and scientific team working on new therapies and approaches for UTUC. We individualize a treatment plan for each patient based on cancer characteristics, and their other medical issues that may impact tolerance to treatment, that can include standard as well as cutting edge experimental approaches. As there are characteristics of UTUC that appear different than bladder urothelial cancer, we continue to work to understand how these diseases differ and to investigate novel therapies to improve the lives of patients with UTUC, and all patients with urothelial cancers.
-
Trial Spotlight Video: The EA8192 Study Team Discusses Their Trial for High Grade Upper Tract Urothelial Cancer
Study chair Jean Hoffman-Censits, MD, and co-chairs Petros Grivas, MD, PhD (Univ. of Washington), and Vitaly Margulis, MD (UT Southwestern Medical Center), review the goals and potential impact of EA8192. Noah Hahn, MD, moderates the discussion.
-
A Growing Understanding and Multidisciplinary Approach to Treating Upper Tract Urothelial Carcinoma
UTUC clinic co-director Jean Hoffman-Censits, MD joins UroToday to highlight multidisciplinary work in upper tract urothelial carcinoma, and to discuss the current treatment landscape for high-grade UTUC.

-
In-person clinical appointments
We offer in person clinical visits here at the Johns Hopkins Hospital in Baltimore, MD, USA. We are unable to provide consultations via telemedicine or remote medical opinions. Click here to contact us for more information.

Jeannie Hoffman-Censits, MD

- Co-Director, Upper Tract Urothelial Cancer Multidisciplinary Clinic
- Associate Professor of Oncology
UTUC Clinical Trials
The goal of a clinical trial is to find a better way to treat upper tract urothelial cancer and help patients. The trials test various treatment options, including new drugs, new approaches to surgery or radiation therapy, new combinations of treatments, or new methods, such as gene therapy. Learn more about our current and ongoing UTUC clinical trials below.
Open UTUC Clinical Trials
Clinical trials currently recruiting patients at the Johns Hopkins Greenberg Bladder Cancer Institute
NCT04620239: ENdoluminal LIGHT ActivatED Treatment of Upper Tract Urothelial Cancer (ENLIGHTED) Study (UCM301)
This is a phase 3, open label, single arm study of padeliporfin in the treatment of Upper Tract Urothelial Carcinoma (UTUC). The ENLIGHTED study will recruit patients with low-grade non-invasive upper tract urothelial carcinoma in either the kidney or the ureter. Patients will be treated with padeliporfin VTP in two phases: an Induction Treatment Phase and a Maintenance Treatment Phase and will be followed up for up to an additional 48 months in the long term (non intervention) follow up phase with the specific duration depending on the patient's response to treatment.
NCT04628767: Testing the Addition of MEDI4736 (Durvalumab) to Chemotherapy Before Surgery for Patients With High-Grade UTUC
This phase III trial compares the effect of adding durvalumab to chemotherapy versus chemotherapy alone before surgery in treating patients with upper urinary tract cancer. Immunotherapy with monoclonal antibodies, such as durvalumab, may help the body's immune system attack the cancer, and may interfere with the ability of tumor cells to grow and spread. Chemotherapy drugs, such as methotrexate, vinblastine, doxorubicin, cisplatin, and gemcitabine work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Durvalumab in combination with chemotherapy before surgery may enhance the shrinking of the tumor compared to chemotherapy alone.
NCT04197986: Study of Oral Infigratinib for the Adjuvant Treatment of Subjects With Invasive Urothelial Carcinoma With FGFR3 Alterations
This is a Phase 3 multicenter, double-blind, randomized, placebo-controlled study to evaluate the efficacy of infigratinib (an oral targeted FGFR1-3 inhibitor) versus placebo, as adjuvant treatment following surgery in adult subjects with invasive urothelial carcinoma and susceptible FGFR3 genetic alterations (mutations, and gene fusions or rearrangements) who have disease that is considered at high risk for recurrence with surgery alone. The study enrolls subjects with either bladder cancer post radical cystectomy or upper tract urothelial cancer post distal ureterectomy and/or nephrectomy. Study treatment is randomized 1:1 between infigratinib or placebo with treatment up to 1 year or until invasive local, distal, or metastatic disease recurrence confirmed by independent imaging reviewer.