Biology of Healthy Aging Program
Studies within this group range from molecular and cellular to genetic and laboratory animal-based approaches targeted to understanding the mechanisms, risk factors, and pathophysiology of aging-related changes in health, function, and quality of life.
In addition, the faculty and staff involved in this interdisciplinary program has considerable expertise in the development of clinical translational studies in human populations, epidemiological studies in populations of older adults, and in clinical intervention trials.
The major aims of the Biology of Healthy Aging Program
Investigate etiologies for age-related activation in inflammatory and renin-angiotensin system pathways and their consequences on the development of frailty, sarcopenia, cognitive decline, osteopenia, and other chronic diseases of older adults.
Develop treatments that focus on inflammation and angiotensin system activation and their consequences.
Investigate the viral-related causes and treatments for immunosenescence in older adults, and develop treatments for these etiologies.
Develop novel vaccination strategies for frail older adults for influenza and CMV.
Study the etiologies and consequences of aging-related changes in mitochondria on health and well being.
Develop and validate physiotypes of frailty and robust health in older adults by integrating genetic, cellular, and clinical level phenomena into integrative biological models
Translate novel aging-related biological findings into diagnostic tests, novel treatments, and preventive strategies that will help to optimize quality of life and healthy aging.
Our Faculty
Peter Magdy Abadir, M.D.
Associate Professor of Medicine

Tae Hwan Chung, M.D.
Assistant Professor of Physical Medicine and Rehabilitation
Assistant Professor of Neurology

Neal S. Fedarko, Ph.D.
Director, Clinical Research Core Laboratory, Institute for Clinical & Translational Research
Co-Director, Fellowship Training Program in Gerontology and Geriatrics
Co-Director, Biology of Healthy Aging Program, Johns Hopkins School of Medicine
Professor of Medicine

Sean Xiao Leng, M.D., Ph.D.
Professor of Medicine

Lolita Sai Nidadavolu, M.D., Ph.D.
Assistant Professor of Medicine

Esther Seunghee Oh, M.D., Ph.D.
Co-Director, Johns Hopkins Memory and Alzheimer's Treatment Center
Associate Professor of Medicine
Assistant Professor of Pathology
Joint Appointment in Psychiatry and Behavioral Sciences

Jeremy David Walston, M.D.
Raymond and Anna Lublin Professor of Geriatric Medicine & Gerontology
Deputy Director, Division of Geriatric Medicine and Gerontology
Co-Director, Biology of Healthy Aging Program
Professor of Medicine
Professor of Oncology

Reyhan Marcus Westbrook, Ph.D.
Assistant Professor of Medicine

Our Fellows
Mariann Gabrawy

Gizem Keceli

Thomas Laskow, M.D.

Geoffrey Shimberg

The Aging Renin Angiotensin System
The Renin Angiotensin System (RAS) is a major hormone system with a crucial role in physiologic and pathophysiologic mechanisms in almost every organ system, including brain, liver, kidney, bones, heart, and blood vessels. It is regarded as a key regulator of hypertension, cardiovascular disease, and renal function. Evidence strongly supports that Angiotensin II (Ang II) promotes inflammation and the generation of reactive oxygen species and governs the onset and progression of vascular senescence, which are all associated with the functional and structural changes that lead to age-related diseases.
-
At the Johns Hopkins Division of Geriatric Medicine and Gerontology, researchers are focusing on understanding the effect of age-related changes in the renin angiotensin system as a critical part of the aging process and a potential cause for age-related diseases.
The objective of the research is to evaluate relationships between AT1R and AT2R in immune system cells as people age and determine how these changes may influence chronic inflammation, frailty, and late-life vulnerability. Genetic and hormonal features that may contribute to the development of chronic inflammation via the angiotensin receptors' pathway will also be identified. Angiotensin receptor autoantibodies and their role in chronic disease propagation in older adults are under active investigation.
Researchers use state-of-the-art facilities and core labs that include high-resolution transmission and immune electron microscopes, confocal and light microscopes, mesoscale multiplex system, and mCT for small animals imaging.
-
Researchers are pioneering research that spans the gamut from basic pathophysiology experiments in cell cultures to genetically engineered animal models that lack AT1 or AT2 receptors, to clinical human studies aimed at elucidating the role of angiotensin II type 1 and 2 receptors in aging, using blockers to the system.
-

Peter Magdy Abadir, M.D.
Associate Professor of Medicine
Neal S. Fedarko, Ph.D.
Director, Clinical Research Core Laboratory, Institute for Clinical & Translational Research
Co-Director, Fellowship Training Program in Gerontology and Geriatrics
Co-Director, Biology of Healthy Aging Program, Johns Hopkins School of Medicine
Professor of Medicine
Jeremy David Walston, M.D.
Raymond and Anna Lublin Professor of Geriatric Medicine & Gerontology
Deputy Director, Division of Geriatric Medicine and Gerontology
Co-Director, Biology of Healthy Aging Program
Professor of Medicine
Professor of Oncology
Chronic Inflammation and Late-Life Decline

The importance of age-related, low-level chronic elevations in inflammatory cytokines is increasingly recognized as pathophysiologic in older adults. This chronic inflammatory state has adverse effects on multi-organ systems, leading to the development of chronic diseases, disability, frailty, and mortality in older adults.
-
The research at Johns Hopkins Division of Geriatric Medicine and Gerontology attempts to delineate the underlying molecular and immune mechanisms of chronic inflammation as well as its potential role in the development of frailty and functional decline in older adults.
-
- The role of chronic inflammation in the pathogenesis of frailty and sarcopenia
- Activation of the monocyte/macrophage-mediated inflammatory pathway in aging and frailty
- Chronic cytomegalovirus (CMV) infection and its role in the development of chronic inflammation and frailty in older adults
- Chronic inflammation and functional decline in aging HIV/AIDS population
- The development of a serum inflammatory mediator panel that can be used to assess for risk for adverse outcomes in disease states and during surgical procedures in older adults
- The development of mouse models that develop chronic inflammatory pathway activation and the study of how inflammation impacts chronic disease development and the development of frailty
-
Collaborators

Karen Bandeen-Roche, Ph.D.
Hurley-Dorrier Professor and Chair, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health
Deputy Director, Johns Hopkins Institute for Clinical and Translational Research
Director, Epidemiology and Biostatistics of Aging Training Program, Johns Hopkins Bloomberg School of Public Health
Interim Director, Center on Aging and Health
Joint Appointment in Medicine
Luigi Ferrucci, M.D., Ph.D.
Chief of the Longitudinal Studies Section at NIA and the Director of the Baltimore Longitudinal Study on Aging
Ravi Varadhan, Ph.D.
Professor of OncologyFaculty Investigators

Sean Xiao Leng, M.D., Ph.D.
Professor of Medicine
Jeremy David Walston, M.D.
Raymond and Anna Lublin Professor of Geriatric Medicine & Gerontology
Deputy Director, Division of Geriatric Medicine and Gerontology
Co-Director, Biology of Healthy Aging Program
Professor of Medicine
Professor of Oncology
Immune Senescence
Immune senescence, or the aging of the immune system, particularly its effect on changes in lymphocyte development and function, predisposes older adults to a higher risk of latent virus reactivation. The varicella-zoster virus, for example, the agent responsible for chickenpox in children, remains dormant in nerve cells in the trigeminal and dorsal root ganglia and reactivates in later life to cause shingles and post-herpetic neuralgia.
Other persistent viral infections include Epstein-Barr virus, herpes simplex virus-1 and -2, and cytomegalovirus (CMV).
Age-related immune senescent remodeling likely involves both the innate and adaptive immune system and may contribute to the immune system’s functional decline; chronic inflammatory state; and risk for frailty, chronic disease, and functional decline in older adults.
-
Researchers in the Johns Hopkins Division of Geriatric Medicine and Gerontology are focused on understanding the basic biological and physiological changes related to aging and frailty in the human immune system, with a special emphasis on the translational aspects of immunosenescence that have the most relevance to frailty and late-life decline.
-
- Vaccine-induced immune protection against influenza and its underlying mechanisms in frail older adults
- Persistent viral infection and age-related restriction of T cell repertoire diversity
- T cell phenotypic remodeling in aging and frailty
- Innate immune remodeling and monocyte/macrophage activation
- Aging and HIV
-
Collaborators
 Joseph B. Margolick, M.D., Ph.D.
Joseph B. Margolick, M.D., Ph.D.
Joint Appointment in Medicine
Joint Appointment in OncologyFaculty Investigators
 Sean Xiao Leng, M.D., Ph.D.
Sean Xiao Leng, M.D., Ph.D.
Professor of Medicine
Mitochondrial Biology
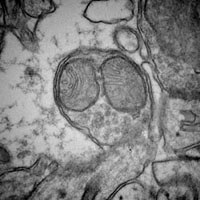
Mitochondria are the cells’ powerhouses and play a crucial role in the development of increased levels of oxidative stress and ultimately in program cell death (apoptosis). Investigators focused on aging have long hypothesized that mitochondria are of crucial importance to the evolution of aging and frail phenotypes.
-
Researchers in the Johns Hopkins Division of Geriatric Medicine and Gerontology are seeking to understand the effect of mitochondrial injury as a critical part of the aging process and a potential cause for age-related diseases.
Currently, researchers are conducting studies that span the gamut from basic pathophysiology experiments in isolated mitochondria to clinical human studies aimed at elucidating the role of mitochondrial damage in aging.
They use state-of-the-art facilities and core labs that include high resolution transmission and immune electron microscope, confocal and light microscopes. An XF96 Extracellular Flux Analyzer allows researchers to simultaneously measure the two major energy yielding pathways, aerobic respiration and glycolysis.
-
- Identification of pathological changes in mitochondria related to the renin-angiotensin system and mitophagy abnormalities and how these aging-related changes may ultimately impact frailty in late life decline
- Mitochondria, mitophagy, and late-life decline
-

Peter Magdy Abadir, M.D.
Associate Professor of Medicine
Jeremy David Walston, M.D.
Raymond and Anna Lublin Professor of Geriatric Medicine & Gerontology
Deputy Director, Division of Geriatric Medicine and Gerontology
Co-Director, Biology of Healthy Aging Program
Professor of Medicine
Professor of Oncology
Muscle and Bone Biology in Frailty
Decreases in physical stature and mobility, which reflect changes in the scaffolding and leveraging systems of the body-the skeleton and muscles, are two easily observed changes associated with aging. Bone and muscle are dynamic organs that undergo continuous repair and remodeling throughout life. These two systems are inter-related because the action of muscle on bone is key to normal remodeling of bone and muscle mass is influenced by bone density.
-
Researchers are focusing on the role of inflammation and autophagy in muscle decline, on biomarker discovery related to this process, and on the role of macrophages in osteopenia.
-
- Repair and renewal pathways that involve genetic influences on muscle strength and quality
- Loss of muscle (sarcopenia) and bone (osteopenia/osteoporosis) which contribute to reduced strength, activity, and energy expenditure leading to disability and dependency
- Genetic influences on muscle mass/strength
- Muscle cell apoptosis and mitochondrial metabolism
- Altered muscle and bone in the IL-10 knockout and other mouse models
- Deficits in mesenchymal stem cell/progenitor cell number and commitment
- Depots of tissue resident senescent macrophages and systemic pro-inflammatory state in a mouse model of osteopenia
-

Peter Magdy Abadir, M.D.
Associate Professor of Medicine
Neal S. Fedarko, Ph.D.
Director, Clinical Research Core Laboratory, Institute for Clinical & Translational Research
Co-Director, Fellowship Training Program in Gerontology and Geriatrics
Co-Director, Biology of Healthy Aging Program, Johns Hopkins School of Medicine
Professor of Medicine
Jeremy David Walston, M.D.
Raymond and Anna Lublin Professor of Geriatric Medicine & Gerontology
Deputy Director, Division of Geriatric Medicine and Gerontology
Co-Director, Biology of Healthy Aging Program
Professor of Medicine
Professor of Oncology
Persistent Viral Infections and T-Cell Repertoires in Older Adults
In older adults, persistent infections caused by herpes viruses can be a common problem. These viruses usually enter the hosts, cause primary disease earlier in life, and remain latent in various cell types. Under the appropriate conditions, these viruses may reactivate to cause disease and elicit recall responses from the adaptive immune system, resulting in inflammation and proliferation of antigen-specific T-cells aimed at suppressing viral replication.
Reactivation of the herpes viruses is more common in immunosuppressed or immunocompromised people who have impaired T-cell immunity, such as transplant recipients and patients infected with the human immunodeficiency virus (HIV).
Human cytomegalovirus (CMV) infects more than 80 percent of adults 60 years and older in the United States. After an acute infection that may produce anywhere from no symptoms in young healthy adults to severe congenital syndromes in newborns, CMV remains latent in mononuclear cells, among other cell types, in a nonreplicating or slowly replicating form.
In immunocompromised patients, CMV is the most common opportunistic pathogen to cause significant morbidity and mortality, reactivating frequently and producing severe disease. However, the long-term clinical effect of CMV infection in people with a competent immune system is not well known. Cardiovascular disease has been associated with CMV seropositivity in adults.
-
In a recent study involving participants from the Women’s Health and Aging Studies, Johns Hopkins researchers showed for the first time that CMV seropositivity leads to a higher five-year mortality in immunocompetent older women, and that women with the highest CMV IgG antibody concentrations are at the highest mortality risk.
Employing novel technologies in T-cell repertoire analysis, researchers in the Division of Geriatric Medicine and Gerontology are dissecting the T-cell response to CMV infection in order to better understand the basis of its pathogenesis in older adults. Given the changes in T-cell repertoire diversity with aging, these research endeavors will also provide important insights into immune protection in older age and influence strategies to improve vaccine effectiveness in older adults.
-
- Antigen-specific T-cell receptor repertoires in humans
-

 Peter C. Doherty, PhD
Peter C. Doherty, PhD