Research Story Tip: Down Syndrome Mice Open Door to Better Understanding of the Genetic Disorder
08/18/2020
Scientists across the globe often use mouse models in the study of human conditions to advance the pursuit of medicines and treatments. Now, Johns Hopkins Medicine researchers and their collaborators have created and characterized a new mouse replica of Down syndrome, long considered one of the most challenging disorders to simulate in laboratory animals.
A report of their research appeared June 29, 2020, in the journal eLife Sciences.
The new model may help researchers better understand how people with Down syndrome learn and develop, and eventually, lead to new therapies for potentially deadly complications of the condition, such as heart disease and thyroid problems.
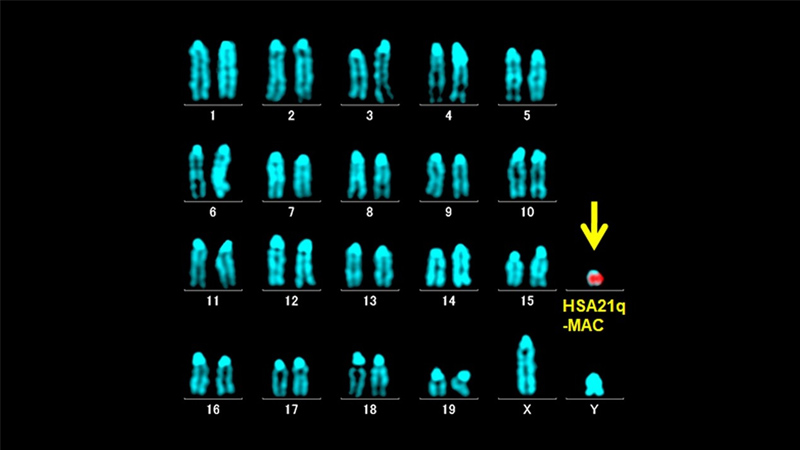
Down syndrome, caused by trisomy 21, occurs when a person is born with an extra partial or entire copy of the 21st chromosome. Typically associated with distinct facial features and developmental delays, people with Down syndrome also experience difficulties with learning and memory, as well as higher rates of thyroid disease, blood and immune disorders and heart disease. Treating these conditions in people with Down syndrome is complicated by their genetics.
“There are more than 500 genes on chromosome 21 that can be overexpressed,” says Roger Reeves, Ph.D., professor of physiology at the Johns Hopkins University School of Medicine. “So, in comparison to many other genetic conditions, Down syndrome is vastly more complex.”
Further complicating the development of successful treatments is the lack of an accurate animal model to study the biology of Down syndrome and test potential therapies for conditions associated with it.
Reeves and his team endeavored to improve research efforts by creating a more precise replica of the condition in mice. They did this by inserting a human copy of chromosome 21 into mice using the rodent’s own cellular structures that organize DNA. This enables the mouse cells to reliably copy and sort the extra human chromosome into new cells as they divide. It also lets the mice pass the additional genetic material on to their offspring.
This means that these mice, named TcMAC21 by the researchers, can be used relatively easily and at low cost in long-term experiments.
The resulting TcMAC21 mice have many characteristics indicative of Down syndrome in humans, including a distinct facial structure, a greater prevalence for congenital heart defects, a smaller-than-usual cerebellum and learning difficulties.
The researchers caution that no single animal model can perfectly replicate a human condition. However, they believe that the TcMAC21 mouse model developed in this study is a good starting point to create new and better strategies for helping people with Down syndrome.
“Our goal is to improve the health of people with Down syndrome to give them the best chance at achieving their full potential,” says Reeves.
