Hopkins-Led Team Finds Biomarkers to Diagnose Serious Kidney Allergic Reaction
05/16/2019
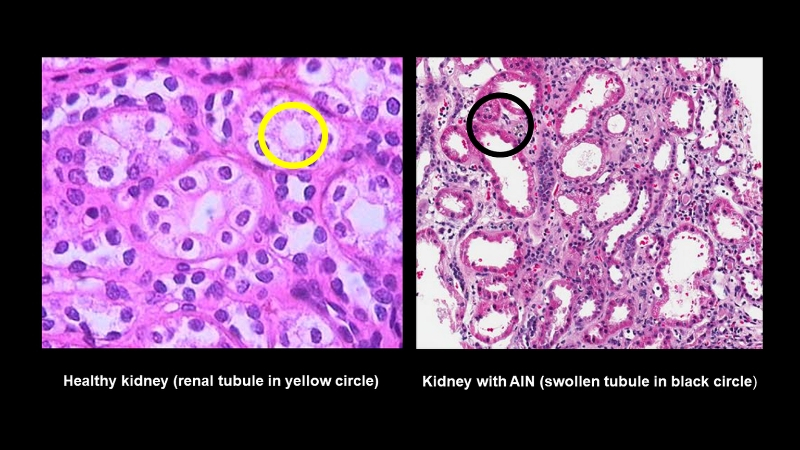
A team led by Johns Hopkins Medicine researchers says it has identified two protein biomarkers in urine that may one day be used to better diagnose acute interstitial nephritis (AIN), an underdiagnosed but treatable kidney disorder that impairs renal function in the short term and can lead to chronic kidney disease, permanent damage or renal failure if left unchecked.
The finding is reported in the May 16, 2019, issue of the Journal of Clinical Investigation Insight.
Acute interstitial nephritis is a condition marked by inflammation and swelling of the renal tubules, the tiny portals in the kidneys where blood is filtered. As a result, the tubules cannot properly reabsorb water and useful organic substances, such as glucose and amino acids, or secrete waste products such as urea and creatinine into urine. AIN is commonly the result of autoimmune diseases or allergic reactions to more than 100 medications, including antibiotics, pain relievers and antacids. The disease is estimated to cause 15 to 20% of all hospitalizations for acute kidney injury.
Currently, the only method of diagnosing AIN is by examining renal tissue obtained with a biopsy, putting patients at some risk from complications. Additionally, samples can sometimes be misinterpreted or yield inconclusive results.
“What has been needed is a biological diagnostic tool that is safe, easy to get and measure, simple to interpret, consistent across patient populations and most importantly, extremely accurate at identifying AIN.” says Chirag Parikh, Ph.D., director of the Division of Nephrology at Johns Hopkins University School of Medicine and senior author of the new paper.
To find such a biomarker, Parikh and his colleagues looked for a substance linked to AIN’s most distinct characteristic, inflammation of the renal tubules, formally known as tubulitis.
Knowing that cytokines — proteins secreted by immune cells known as CD4+ T lymphocytes — are the agents causing inflammation of the tubules, the researchers measured the amounts of 12 urine and 10 blood plasma proteins in samples from 79 adult, biopsy-confirmed AIN patients, and compared them to the amounts in 186 adult kidney biopsy patients without an AIN diagnosis.
The results, reviewed independently by three pathologists, showed that none of the plasma biomarkers were associated with AIN, but that two proteins — tumor necrosis factor-alpha (TNF-α) and interluken-9 (IL-9) — were consistently seen in the urine of AIN patients. Neither cytokine was present in the control samples, either in plasma or urine.
“Because both cytokines are seen in allergic diseases such as atopic dermatitis and food allergies, and AIN most often is the result of an allergic response, it makes sense to consider using them as diagnostic tools,” Parikh says.
“Additionally, we knew that IL-9 leads to the accumulation of mast cells that release histamine and other chemicals that induce allergic responses, and kidney biopsies from AIN patients frequently reveal the presence of mast cells,” he adds. “This suggests that IL-9 may be the stronger link to AIN, and perhaps, the better candidate as a future predictive biomarker.”
To test the sensitivity of using IL-9 in this manner, the researchers compared its AIN-detecting ability in patients whose disease was confirmed by pathologists who evaluated biopsied material or was symptomatically diagnosed by clinicians prior to the biopsies.
“If biopsy diagnoses are considered 100% accurate, then IL-9 had a very comparable ranking of 84%, and this was significantly better than the 62 to 69% achieved by clinicians without biopsies,” Parikh says.
The next step for the research team, Parikh says, will be to verify the findings of this study in a larger group of patients and get support for the development of TNF-α and IL-9 as tools to complement AIN diagnosis by biopsy. He says the hope is that later, biomarkers can replace kidney biopsies altogether.
“Biopsy is currently the ‘gold standard’ for AIN diagnosis, but we believe that biomarkers will one day be a more robust, more accurate, more cost effective and more patient-friendly method,” Parikh says.
Along with Parikh, the other Johns Hopkins University School of Medicine (Baltimore, Maryland) author on the paper is Wassim Obeid, Ph.D. Authors from the Yale School of Medicine (New Haven, Connecticut) include the lead on the paper, Dennis Moledina, Ph.D.; and his colleagues Lloyd Cantley, M.D.; Randy Luciano, M.D., Ph.D.; Michael Kashgarian, M.D.; Gilbert Moeckel, M.D., Ph.D.; Mark Perazella, M.D.; Jordan Pober, M.D., Ph.D.; Nikhil Singh, M.D., Ph.D.; and F. Perry Wilson, M.D., M.S. Also listed as authors are Haiqun Lin, M.D., Ph.D., of the x Yale School of Public Health; and Michael Kuperman, M.D., of Arkana Laboratories (Little Rock, Arkansas).
Two of the authors, Parikh and Moledina, are named as inventors in a provisional patent number 62/716,465, titled “System and Methods for Diagnosing Acute Interstitial Nephritis.” No other author has any conflict of interest relevant to the study.
