Found! Lost Puzzle Piece Involved in Gene Regulation Revealed in Search That Began in Water-Loving, One-Celled Organism
12/22/2022
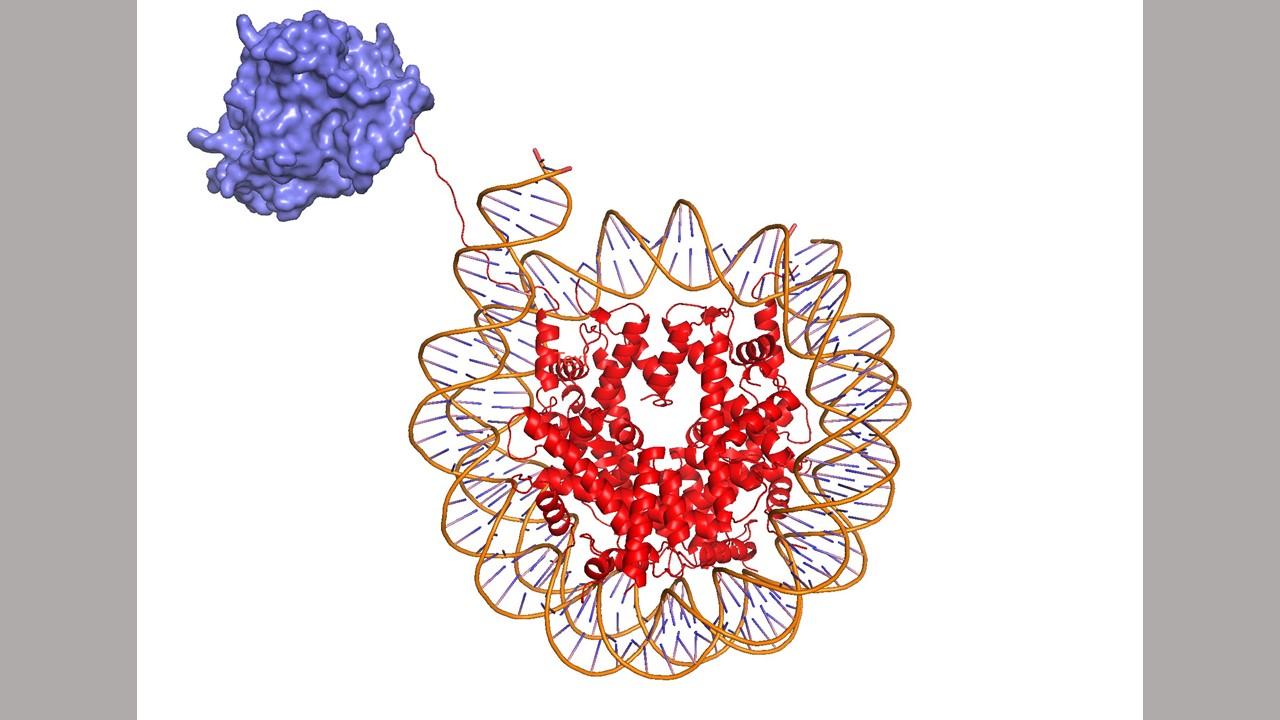
After an intrepid, decade-long search, Johns Hopkins Medicine scientists say they have found a new role for a pair of enzymes that regulate genome function and, when missing or mutated, are linked to diseases such as brain tumors, blood cancers and Kleefstra syndrome — a rare genetic, neurocognitive disorder.
The new findings, published Nov. 21 in Epigenetics & Chromatin, could eventually help scientists understand diseases caused by disruption of these enzymes and develop new treatments for them.
“Developing a better understanding of how enzymes impact the activity of our genomes offers valuable insights into biology and can help researchers design new therapeutic approaches for disease,” says Sean Taverna, Ph.D., associate professor of pharmacology and molecular sciences at the Johns Hopkins University School of Medicine.
The search began more than a decade ago, when Taverna was looking for factors that influence DNA activity in Tetrahymena thermophila — a one-celled, fresh water dwelling organism. During the original study, the research team found a previously unknown signal that the single-celled creature uses to “mark” genes it has turned off.
The location of the mark is on histone proteins, which act as spools that tightly wind DNA, often turning off genes and protecting DNA from damage. If Tetrahymena are not able to add the marks — a process called methylation, which adds chemical tags to a part of histones called H3K23 — the DNA becomes damaged and the cells grow poorly.
In a follow up study published in 2016, Taverna found that the H3K23 location is conserved between Tetrahymena and mammals, including humans. However, the enzymes that control how the chemical tags are placed on H3K23 differ between the species.
Without the identity of these enzymatic H3K23 “writers” of methylation, the researchers found it difficult to study H3K23’s role in human biology and disease.
So, Taverna, recent Ph.D. graduate David Vinson and Srinivasan Yegnasubramanian, M.D., Ph.D., professor of oncology and pathology at the Johns Hopkins Kimmel Cancer Center, led a new study to search for the mammalian enzymes that add the chemical tags to H3K23.
After screening many enzymes that write methylation, Vinson found just one pair of enzymes, EHMT1/GLP and EHMT2/G9a, which placed chemical tags on the H3K23 histone location.
When the researchers used drug inhibitors and genetic mutations directed against the enzyme pair in human brain cells (neurons) grown in the laboratory, the ability of the enzymes to place methylation tags on the H3K23 histone location reduced significantly.
“With this initial precedent established in human neuronal cells, the door is now wide open to study the role of these enzymes and the H3K23 modification in numerous contexts of health and disease, including human cancer,” says Yegnasubramanian.
Now that the researchers know that EHMT1/GLP and EHMT2/G9a place chemical tags on the H3K23 histone location, they are aiming to understand the precise mechanism of how they do so and develop drugs that target this activity.
“We want to better understand why diseases occur when these enzymes aren’t working correctly, and what their connections are to H3K23,” says Taverna.
In addition to Taverna, Yegnasubramanian and Vinson, other researchers who contributed to the study are Kimberly Stephens of the University of Arkansas for Medical Sciences; Robert N. O'Meally, Shri Bhat and Robert Cole of Johns Hopkins; and Blair Dancy of the Walter Reed Army Institute of Research.
Funding for the study was provided by the National Institutes of Health (R01GM118760, R01CA221306 and F31GM130114), the National Science Foundation, the Irving A. Hansen Memorial Foundation and the Commonwealth Foundation.
