Contact Us
The Office of Human Subjects Research (OHSR) is a fully remote office. Staff can be reached via phone, email, or MS Teams chat. Contact information for each staff member is listed below.
For General IRB, eIRB Technical Assistance, and Training Questions:
For questions about IRB processing, eIRB technical questions or training questions you should contact the IRB Help Desk. The most efficient way to contact the Help Desk is through email. You can also call the general voicemail number and leave a message. Someone will return your call as soon as possible. Calls and emails that cannot be addressed directly with the Help Desk are routed to an appropriate OHSR staff member.
IRB Help Desk E-Mail: [email protected]
IRB Help Desk Phone: 410-502-2092
Request a Consult
If you require a more comprehensive discussion (e.g. you need assistance in protocol planning, data sharing), please request a consult by clicking on this link. A virtual meeting with a member of our staff will be scheduled using MS Teams.
Questions About Specific Protocols or Application Processing
Click on the links below to quickly find the contact information that best fits your situation.
- Pre-Team IRB Coordinator/Analyst - For questions regarding when an application will be scheduled for review, issues returned in a pre-review note and placement of documents in the application.
- Post Team IRB Coordinator/Analyst - For questions regarding the outcome of a study, requesting an extension to respond, stamped documents, questions about tabled issues or questions about your outcome letter.
- Consent Form Specialists - For questions about consent forms, the consenting process or types of consent please contact one of the consent form specialists.
- Compliance Associates - For questions about regulatory issues, compliance with federal, state and local policies, general compliance issues, protocol events or noncompliance.
- Reliance Team - For questions about requesting a reliance agreement, single IRBs for a multi-center study, or relying on a commercial IRB please contact the IRB Reliance Program.
- Biospecimen Program Administrator - For questions about transferring biospecimens outside of Hopkins, human subjects research utilizing biospecimens, or research involving stem cells, please contact the Biospecimen Program Administrator.
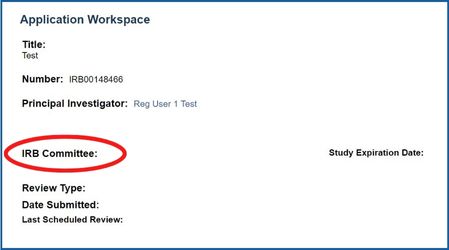
Not sure what committee your application is with? Log into eIRB2 and open up the Application. This will direct you to the application workspace page and you will be able to see the committee your application has been assigned to.
| Office of Human Subjects Research Staff Contact Information | ||
|---|---|---|
| Megan Singleton, JD, MBE, CIP | Associate Dean for Human Research Protection and Director of the Human Research Protection Program | [email protected] 443-927-1489 MS Teams Chat |
| Kat Jeter | Sr. Administrative Coordinator | [email protected] 443-927-1466 MS Teams Chat |
| Operations | ||
| Barbara Scherer, MAS, CIP | Director of Operations | [email protected] 443-927-1487 MS Teams Chat |
| Seth Hall, JD | Associate Director OHSR Operations | [email protected] 443-927-8032 MS Teams Chat |
| Pre-Team IRB Analysts & Coordinators | ||
| Veronica Walters Pre-Team IRB 1 |
IRB Analyst | [email protected] 443-927-1496 MS Teams Chat |
| Andrew Tomer Pre-Team IRB 2 |
IRB Analyst | [email protected] 443-927-1494 MS Teams Chat |
| Sophie Klein Pre-Team IRB 3 |
IRB Coordinator | [email protected] 443-927-1470 MS Teams Chat |
| William Duck Pre-Team IRB 5 |
IRB Analyst | [email protected] 443-927-1462 MS Teams Chat |
| Sharon Bumbray Pre-Team IRB 6 |
IRB Analyst | [email protected] 443-927-1459 MS Teams Chat |
| Holly Thompson Pre-Team for Changes in Research IRB X |
IRB Coordinator | [email protected] 443-927-1491 MS Teams Chat |
| Juliana Torres Pre-Team for New Applications IRB X |
IRB Analyst | [email protected] 443-927-1495 MS Teams Chat |
| Post-Team IRB Analysts & Coordinators | ||
| Kelly Sniffen Post-Team IRB 1 |
IRB Coordinator | [email protected] 4439271483 MS Teams |
| Mary Szeliga Post-Team IRB 2 |
IRB Analyst | [email protected] 443-927-1492 MS Teams Chat |
| TBD Post-Team IRB 3 |
IRB Coordinator | |
| Jaime Lush Post-Team IRB 5 (Convened) |
IRB Analyst | [email protected] 443-927-1473 MS Teams Chat |
| Lindsay Koenig Post-Team (Expedited) |
IRB Analyst | [email protected] 443-927-1471 MS Teams Chat |
| Kathleen Hand Post-Team IRB 6 |
IRB Coordinator | [email protected] 443-927-1480 MS Teams Chat |
| Katie Sharp Post-Team IRB X |
IRB Analyst | [email protected] 443-927-1488 MS Teams Chat |
| Exempt/Expedited Review | ||
| Kristin MacNeal | Associate Director Exempt/Expedited Review | [email protected] 443-927-1475 MS Teams Chat |
| Tabitha Golda | Exempt/Expedited Compliance Analyst | [email protected] 443-927-1485 MS Teams Chat |
| Tammy Bixby | Exempt/Expedited Review Analyst | [email protected] 443-927-3016 MS Teams Chat |
| JH ACH IRB - Located at Johns Hopkins All Children's Hospital - St. Petersburg, FL | ||
| Ellen Bedard, MSOB, CCRP | JH-ACH IRB Program Manager | [email protected] 727-767-8620 MS Teams Chat |
| Sue Ellie JH-ACH IRB |
IRB Analyst | [email protected] 727-767-4275 MS Teams Chat |
| Consent Form Specialists | ||
| Heather Kammann | Sr. Consent Form Specialist - Lead | [email protected] 443-927-1468 MS Teams Chat |
| Lucas Szylow | Sr. Consent Form Specialist | [email protected] 443-927-1493 MS Teams Chat |
| Mary Medak | Sr. Consent Form Specialist | [email protected] 443-927-1478 MS Teams Chat |
| Lauren Swedberg | Sr. Consent Form Specialist | [email protected] 443-927-1490 MS Teams Chat |
| Kristen Martin | Sr. Consent Form Specialist | [email protected] 443-927-1477 MS Teams Chat |
| Liz Harrop | JH-ACH Consent Form Specialist | [email protected] 443-927-3209 MS Teams Chat |
| Reliance | ||
| Janelle Maddox-Regis, MS | Associate Director IRB Reliance Program | [email protected] 443-927-1476 MS Teams Chat |
| Pamela Ritzler | IRB Reliance Analyst | [email protected] 443-927-1465 MS Teams Chat |
| Mathilda Barnes | IRB Reliance Analyst | [email protected] 443-927-1457 MS Teams Chat |
| TBD | IRB Reliance Coordinator | |
| Compliance | ||
| Ken Borst, JD | Director of Compliance | [email protected] 443-927-1458 MS Teams Chat |
| Kon Kim, JD | Associate Director, Compliance | [email protected] 443-927-1469 MS Teams Chat |
| Eunhae Gohng, JD | Sr. Human Research Compliance Associate | [email protected] 443-927-1463 MS Teams Chat |
| Andrew D. Michalek, MHA, LLM | Human Research Compliance Associate | [email protected] 443-927-1482 MS Teams Chat |
| Luis Beltran | Human Research Compliance Associate | [email protected] 667-306-9264 MS Teams Chat |
| Clemence Miller, JD | Sr. Compliance Advisor | [email protected] 443-927-1479 MS Teams Chat |
| Compliance Monitors | ||
| Frederick W. Luthardt, DBe, MA | Director Compliance Monitoring Program | [email protected] 443-927-1474 MS Teams Chat |
| Suzanna Roettger, MA | Associate Director Compliance Monitoring Program | [email protected] 443-927-1486 MS Teams Chat |
| Margaret Leathers, MS | Sr. Compliance Monitoring Specialist | [email protected] 443-927-1472 MS Teams Chat |
| Cierra Noel, BS | Sr Compliance Monitoring Specialist | [email protected] 443-927-1481 MS Teams Chat |
| Anastasiya Pronina | Compliance Monitoring Specialist | [email protected] 443-927-1484 MS Teams Chat |
| eIRB, Training & Technical Support | ||
| Jessica Jones | Education and Training Manager | [email protected] 443-927-1467 MS Teams Chat |
| Jonathon Harris | Sr Systems Administrator | [email protected] 443-927-1464 MS Teams Chat |
| David Baker | Technical Support Analyst | [email protected] 443-927-1718 MS Teams Chat |
| Billing | ||
| Alisha Wells | Budget Analyst | [email protected] 443-927-1461 MS Teams Chat |
| Biospecimen/ISCRO | ||
| Jessica Williams, MA, MS, CIP | Biospecimen Program Administrator | [email protected] 443-927-1460 MS Teams Chat |

