COVID-19 Booster Vaccine Study in People with Type 1 Diabetes
Individuals with type 1 diabetes (T1D) can be very sensitive to changes (e.g. diet, exercise, illness), as these can cause profound shifts in sugar levels, potentially leading to hypoglycemia (low sugar), hyperglycemia (high sugar), or diabetic ketoacidosis (DKA). It’s well established that acute inflammation in particular can lead to transient insulin resistance and hyperglycemia among patients with diabetes. Anecdotal reports have described hyperglycemia and/or insulin resistance after COVID-19 vaccine administration; however, this has never been studied in a rigorous fashion.
Clinicians from Johns Hopkins are conducting a research study to see if the COVID-19 Booster Vaccine increases glucose levels or insulin requirements in patients with Type 1 Diabetes.
Eligibility Criteria
- Adult (age>18)
- Type 1 Diabetes
- Fully vaccinated against COVID-19 (either two doses of Moderna/Pfizer or one dose of J&J COVID-19 vaccine)
- Planning on getting a COVID-19 vaccine booster
- Not Pregnant
- Received two COVID-19 Pfizer/Moderna vaccine shots or one J&J vaccine shot
- Not on high-dose steroids (e.g. dexamethasone) or other medications which can interfere with glucose measurements.
The study consists two short clinic visits, 10 days apart. No blood tests or imaging are required as part of this study! We will ask you to wear a Dexcom G6 Pro continuous glucose monitor (CGM) for 10 days. You will continue your self-care for your diabetes (e.g. checking sugar levels and administering insulin) as you normally would. We will ask you to keep a log of your insulin doses during the 10 day study period.
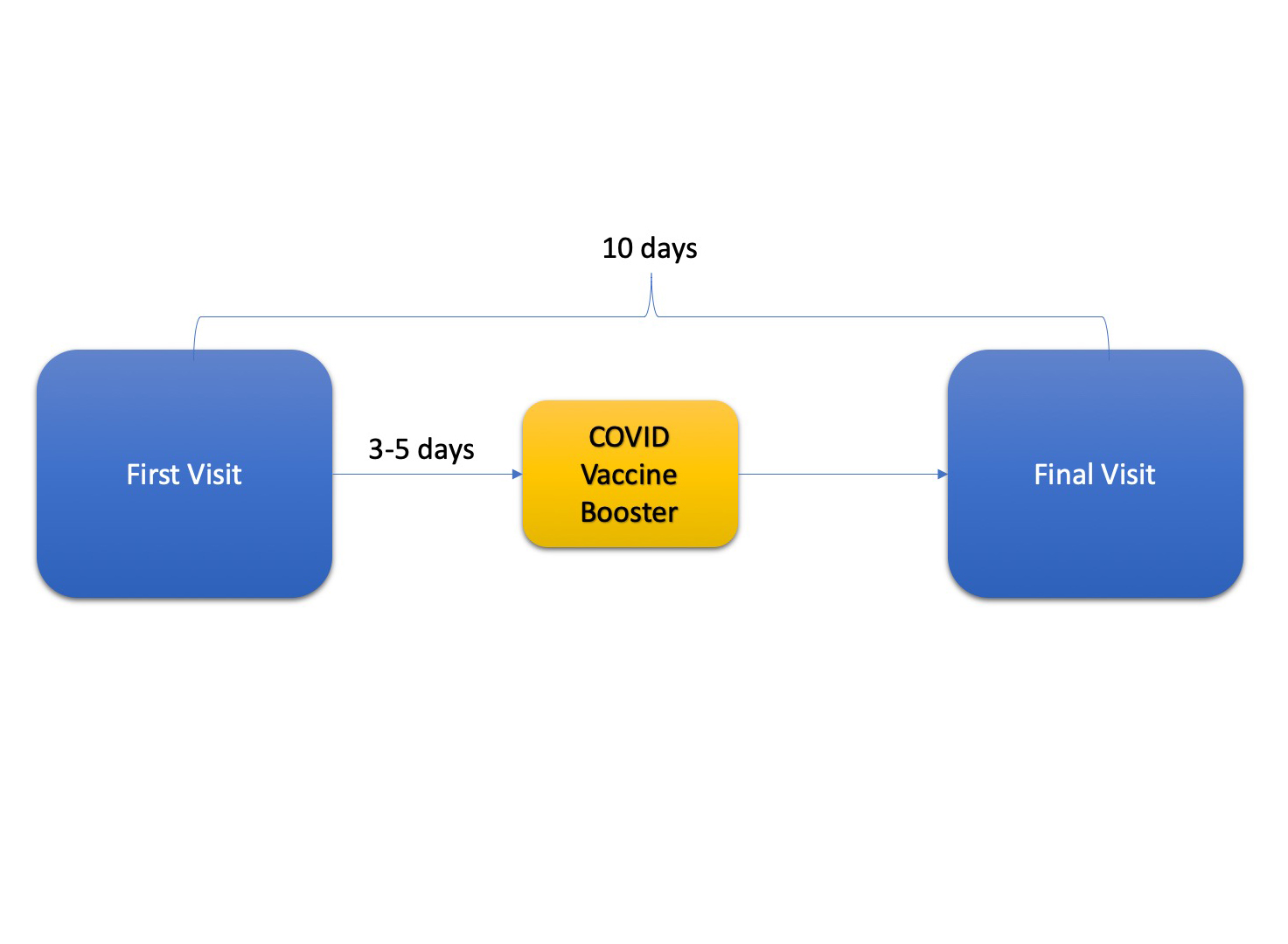
First Visit (approximately 1-2 hours)
Consent form
Physical Exam by a clinician on the study team
Dexcom G6 Pro CGM applied to abdomen/back of the arm
3-5 days later
You get your COVID booster vaccine (at your pharmacy, doctor’s office, workplace, etc)
Final Visit (10 days after the First Visit; ~1-2 hours)
Physical Exam
Bring back your insulin log and proof of COVID-19 booster vaccination
Remove Dexcom G6 Pro CGM
Participants will receive $100 for time and effort in enrolling and completing study visits
Note: We will not be providing or paying for the COVID-19 Vaccine Booster shot as part of this study. Participants have to obtain the booster vaccine on their own, as per usual means (e.g. pharmacy, doctor’s office, workplace, etc).
Study Locations
- Suburban Hospital
- Johns Hopkins Howard County Medical Center campus
For more information, please contact:
Mehro Akhtar
Study Coordinator
[email protected]
443-546-1402
Andrew Demidowich, MD
Principal Investigator
[email protected]
410-720-8238
Study Number: IRB00305567
