Johns Hopkins Medicine to Receive $21.4 Million to Advance Viability of Animal Organs for Transplants and Enable Human Clinical Trials
03/29/2023
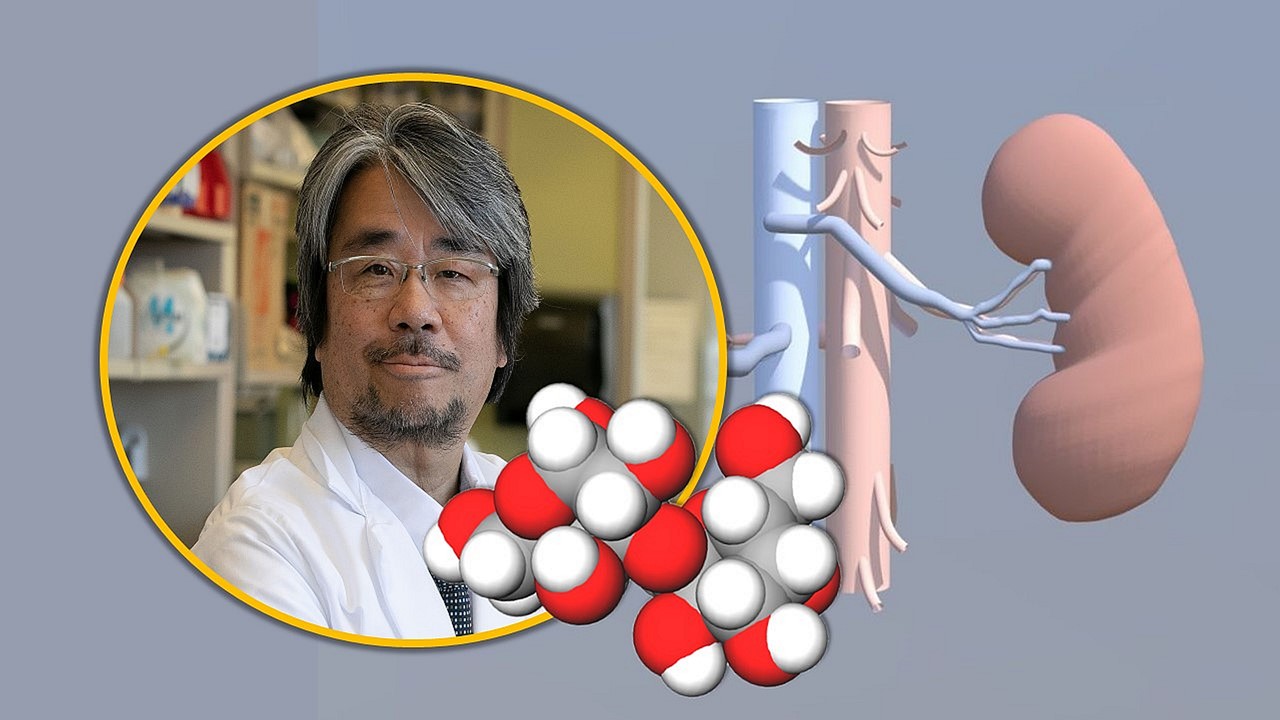
As part of the worldwide effort to facilitate a research and clinical pathway toward successful xenotransplantation — the transplantation of living cells, tissues and organs from one species to another — two Johns Hopkins Medicine surgeons, Kazuhiko Yamada, M.D., Ph.D., and Andrew Cameron, M.D., Ph.D., will receive a total of $21.4 million in funding over the next two years under two sponsored research agreements with biotechnology company United Therapeutics Corporation. The company focuses on developing novel pharmaceutical therapies and technologies that expand the availability of transplantable organs.
The agreements announced today will support preclinical studies (animal and laboratory), conducted in collaboration with United Therapeutics, to advance the use of genetically modified pigs — whose kidneys are more compatible for transplantation into humans than non-modified animals — enabling a reduced risk of immune system attack, better avoidance of organ rejection and failure, and increased chances for a recipient’s long-term survival with the xenograft.
“We are tremendously excited about what we will learn through this new research endeavor at Johns Hopkins Medicine,” says Cameron, surgeon-in-chief and director of the Department of Surgery at the Johns Hopkins University School of Medicine. “Although we have a highly successful kidney transplant program, we’ve been limited — like other medical institutions — by the shortage of available human donor organs. Hopefully, xenotransplantation will soon be able to join other strong efforts at Johns Hopkins to address this challenge, such as our nondirected [altruistic] and directed [designated recipient] living donor programs.”
End-stage kidney disease, a condition that without treatment results in kidney failure and death, can only be remedied by dialysis (mechanically filtering wastes from the blood when the kidneys cannot) or transplantation of a working organ from either a deceased or living donor. Unfortunately, according to the federal government’s Organ Procurement and Transplantation Network, the number of people needing a kidney far exceeds the number of available organs. For example, the website states that in 2022, there were some 96,000 patients on waiting lists for a kidney, but only about 25,500 transplants were performed.
Annually in the United States, the number of usable organs for transplantation remains extremely low, and according to the federal organdonor.gov website, 17 people die each day simply because they cannot get a transplantable human organ.
Researchers worldwide have investigated the use of pig organs — primarily hearts and kidneys — for decades as potential options for xenotransplantation in humans because of similarities between the two species in how their organs function. The U.S. Food and Drug Administration (FDA) has not yet approved such transplants for clinical use, but has on rare occasions permitted “compassionate use” exceptions.
“Over the next two years, our research team hopes to improve the strategies and techniques that have made genetically modified pig kidneys for transplantation so promising to this point, and then take them to the level where human clinical trials can begin,” says Yamada, professor of surgery at the Johns Hopkins University
School of Medicine. “Then, hopefully, we can finally realize that promise.”
Yamada, recruited in August 2022 to lead Johns Hopkins Medicine’s growing xenotransplantation research program, is considered a pioneer in the field. In 2003, he performed the first pig-to-primate kidney xenotransplant using genetically modified alpha-gal knockout (or GaIT-KO) pig kidneys.
Alpha-gal sugar is a compound on cell surfaces that stimulates the immune system, and it is believed to be a trigger of transplant rejection in humans. GalT-KO pigs are modified so that they lack the gene that produces alpha-gal sugar. This modification — known as a gene “knockout” — makes their tissues and organs more likely to be accepted.
“Under the new research agreements,” Yamada says, “we will study the impact on xenotransplantation of knockouts, as well as ‘knocking in’ [adding] human genes that could help prevent rejection.”
A second approach to be studied under the new agreements is to “teach” the human immune system to recognize the donated pig organ as “self.” This involves a technique pioneered by Yamada — concurrently transplanting a pig kidney with thymus tissue from the same animal donor.
“By transplanting pig thymus tissue along with the donor kidney, the immune response of the recipient is reduced, prolonging the viability of the organ, and with less need for medical immunosuppression,” says Yamada.
“With the new research agreements, we can help complete the critical preclinical work in animals that the FDA has requested before the first clinical trials of genetically modified pig kidney xenografts in humans can begin,” explains Cameron. “Those studies in the near future — in which Johns Hopkins Medicine hopes to be involved as a trial site — could potentially lead to xenotransplantation becoming a viable way to help alleviate the nation’s transplant organ shortage, not only for kidneys, but for other organs as well.”