Research Story Tip: Better Repaired Nerve Insulation May Lead to New Multiple Sclerosis Treatments
10/27/2020
Note: To download the image above, click here.
In a new study using mice, Johns Hopkins Medicine researchers have found a better way than natural healing to repair damaged insulation surrounding nerve cells. Normally, the natural healing process adds bumps to the surface of the protective fatty sheath, known as myelin, each time it’s repaired. Over time and after cumulative damage, the myelin ultimately becomes too misshapen to wrap cleanly around the nerve, causing it to lose function.
This happens in multiple sclerosis (MS), a disorder in which the body’s immune system mistakenly attacks the myelin around nerves, shutting them down and causing communication problems between the brain and the rest of the body.
In their study published on Oct. 2, 2020, in Science Advances, the researchers say that using certain drugs may prevent relapsing-remitting MS, the intermittent form of the disorder, from becoming progressive MS — a chronic form of the condition in which the myelin can no longer repair itself.
“Suppressing the immune system has worked to treat relapsing-remitting MS, but it doesn’t protect from the eventual advancement to progressive MS, for which there aren’t any good treatments on the market,” says Norman Haughey, Ph.D., professor of neurology at the Johns Hopkins University School of Medicine. “We think these findings are a big step toward improving the quality and composition of myelin following a flare-up.”
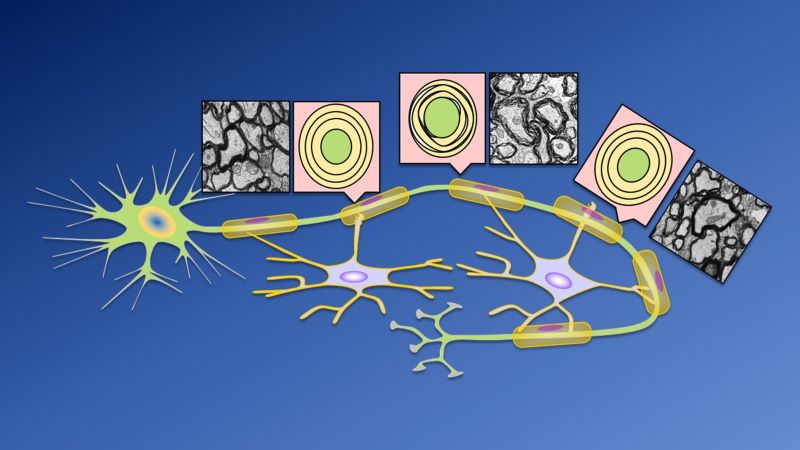
In earlier work by Haughey’s team, the researchers looked at the composition of the myelin surrounding nerves found near injured brain tissue taken from deceased people with MS. Myelin is made mostly from hundreds of types of fat molecules and proteins. The researchers saw that myelin around nerves near injury sites looked misshapen compared with that of other nerves, along with having much higher levels of ceramide — a particular type of fat molecule — and lower levels of another fat molecule called sulfatide.
Having the correct amount of ceramide is especially important because this fat regulates the curvature of myelin — too much ceramide and it can’t wrap tightly around the nerve, creating “bumps” in the myelin.
In the new study, the researchers fed the drug cuprizone to mice for 26 days to damage the myelin on their nerve cells. The myelin repaired itself, but looked it bumpy and wrapped poorly around the nerve because of the excess ceramide. In a series of experiments, the researchers found that brain inflammation activates the enzyme, neutral sphingomyelinase-2, which produces ceramide.
Working with an expert drug development team led by Barbara Slusher, Ph.D., M.A.S., professor of neurology at the Johns Hopkins University School of Medicine, the researchers identified the small molecular size drug, cambinol, which blocks neutral sphingomyelinase-2 from working. They theorized that this would prevent excess ceramide from being made and incorporated into regenerated myelin after an injury.
After nearly a month of feeding their mice cuprizone to cause myelin damage, the researchers injected them with cambinol. When the myelin grew back this time, it wrapped tightly around the neurons and looked like it did before the damage.
The researchers say this intervention did not completely restore the fat composition of myelin, but it appeared to increase the stability of the myelin, which likely would better protect the underlying neurons.
The team needs to determine if there are impacts from other abnormal fat levels in the repaired myelin even with the prevention of excess ceramide buildup. Also, the researchers need to confirm that the myelin — after being in its correct shape and structure — functions as it should and is more stable over long periods of time.
Once this is done, the team hopes to develop small molecular-size inhibitors of neutral sphingomyelinase-2 for eventual use in human trials.
Postdoctoral fellow Seung-Wan Yoo, Ph.D., M.S., is the lead author of this study.