Investigators Devise Test to Identify Brain Tumors from Cerebrospinal Fluid
09/06/2023
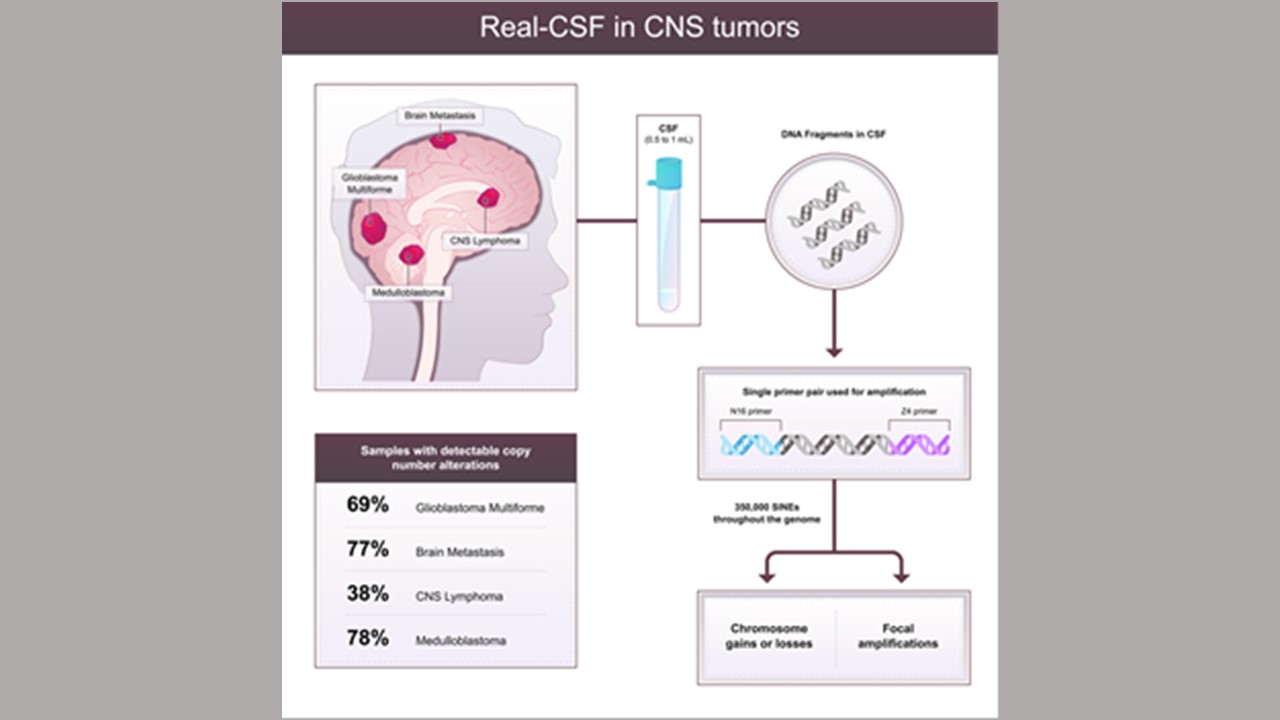
Researchers with the Johns Hopkins Kimmel Cancer Center, the Johns Hopkins University School of Medicine and four other institutions have developed a molecular test to identify the presence of brain tumors by measuring abnormal genetic material shed by tumors and circulating in cerebrospinal fluid (CSF). A description of the work was published Aug.15 in the journal Cell Reports Medicine.
Typically, brain tumors are assessed through MRI imaging and biopsies. The novel test, called Real-CSF (repetitive element aneuploidy sequencing in CSF), assesses aneuploidy (chromosome copy number alterations found in cancers) in over 350,000 regions of the genome simultaneously. A companion bioinformatics algorithm and machine-learning process allows researchers to identify in as little as 2 milliliters of CSF if cancers are present and what molecular characteristics they demonstrate.
In laboratory evaluations of the test in 280 CSF samples from patients, some with brain or other cancers and some without cancer, Real-CSF correctly identified 67% of 184 cancerous brain lesions and 96% of 96 noncancerous lesions. This analysis was more likely to correctly identify cancers than the standard of care, cytology. Of 121 patients with cancer in whom cytology results were available, only 28 (23%) were detected by cytology, whereas Real-CSF correctly detected 69% of cancers in this group.
If validated in additional studies and clinical trials, Real-CSF could be used to help clinicians distinguish between cancerous and noncancerous lesions, and provide information regarding how brain tumors are responding to treatment, says senior study author Chetan Bettegowda, M.D., Ph.D., the Jennison and Novak Families Professor of Neurosurgery and a professor of oncology at the Johns Hopkins University School of Medicine, director of the Metastatic Brain Tumor Center at Johns Hopkins and medical director of the Ludwig Center for Cancer Genetics and Therapeutics.
One of the next steps is to combine the approach with other substances such as mutations in genes associated with cancer, or changes to DNA that are cancer-specific, to improve the test performance, Bettegowda says.
“The test is very simple to use, works even on a limited amount of CSF and is inexpensive relative to many of the other liquid biopsy approaches on the market,” he says. “With those characteristics, we were quite pleased that we had such a robust performance.”
Bettegowda and lead study author Christopher Douville, Ph.D., an assistant professor of oncology at Johns Hopkins, are available for comment. To schedule an interview, contact Valerie Mehl at [email protected] or Amy Mone at [email protected].
