At a time when many high school students are deciding their path in life, Richard Huganir, Ph.D., director of the department of neuroscience, was conducting his first neuroscience experiment, one that would mark the beginning of a decades-long, prolific career.
Years later, Huganir earned his doctorate at Cornell, working in the lab of biochemist Efraim Racker, where he began studying the receptors on brain cells that are activated by neurotransmitters. As a postdoctoral fellow at Yale, Huganir worked with Nobel laureate Paul Greengard, continuing work on the regulation of neurotransmitter receptors. He continued this line of research at his first faculty job at The Rockefeller University.
Then, in 1988, none other than legendary neuroscientist Sol Snyder, for whom the Johns Hopkins Medicine neuroscience department is named, and Nobel laureate Dan Nathans recruited Huganir to join the Johns Hopkins Medicine faculty. That was more than 30 years ago.
Fast forward to last month, when the Society for Neuroscience recognized Huganir’s contributions to the neuroscience field with their highest honor, the Ralph W. Gerard Prize in Neuroscience.
We asked Huganir to recount the milestones of his career and provide his perspective on the future of neuroscience research.
You have been a neuroscientist for more than 40 years. What drew you to this field?
In high school, I was really interested in science. At the same time, I started thinking about what makes me who I am, and a major part of that is my memories. I realized that memories must be encoded by biochemical changes in your brain.
So, for my high school senior research project in biology, I re-created an experiment by famous neuroscientist Bernard Agranoff, in which I trained goldfish to learn a task, then tried to block the learning by using drugs that block protein synthesis. Essentially, by blocking protein synthesis, the fish could not perform the task, suggesting that I was able to block memory formation. This is how I started trying to understand how memories are encoded in the brain, and it’s what I’ve been doing my entire career.
What area of your research has been career-defining?
In my lab, we study how learning is encoded in the brain and maintained for years, or even decades, and for this, we study all kinds of memory.
Learning is a process called synaptic plasticity, in which your brain modifies the connectivity between your brain cells, or neurons. The connectivity between neurons is essentially circuitry, and new connections are made in billions of neurons and quadrillions of synapses, the space between neurons where neurotransmitters pass back and forth before they attach to receptors. When you learn something, you basically sculpt a new circuit by strengthening some synapses and weakening others.
As a graduate student, I had a hunch about the receptors that link to neurotransmitters, and it turned out to be true. They regulate a lot of learning, but in disease, this mechanism breaks down.
For example, in 1998, we identified a protein called SYNGAP, and showed it is involved in normal learning. However, mice with mutations in the SYNGAP1 gene have problems with learning, seizures and hyperactivity.
In 2009, 11 years after our initial discovery, SYNGAP1 mutations were found in children with intellectual disabilities, autistic repetitive features and hyperactivity. It’s one of the most common forms of intellectual disability.
I am now working on developing therapies for children with SYNGAP1 mutations and, with the Kennedy Krieger Institute, starting a clinic focusing on this condition.
This is a basic scientist’s dream, to discover the gene, reveal what it does, and then apply that knowledge to develop therapeutics.
What mentors have been most influential to your career?
Paul Greengard was probably the most important mentor to me. When I first arrived as a postdoc in his lab at Yale, he said, “We’re moving to Rockefeller University.” At the time, I really didn’t want to move to New York. But he believed in me and he convinced me to move with him.
When we got to Rockefeller, he promoted me to assistant professor and, amazingly, gave me the penthouse apartment next door to his, but most importantly, he gave me the independence to create my own track.
At Johns Hopkins, Sol Snyder took me under his wing and groomed me to be the chair of the neuroscience department. I helped with major decisions, led the graduate program for more than a decade and helped build the department over the years, so when he stepped down, he was clear that he prepared me to be chair and, luckily, the director search committee agreed.
Another key mentor was Dan Nathans, who gave me important advice on career development.
In my own mentoring, I try to nurture people and get the best out of each individual by giving them flexibility and encouragement to be creative. That’s what Sol and Dan did for me, and it’s why the projects in my lab seem very diverse.
When you started your career, where did you see the future of neuroscience, and where do you see it now?
When I began my career, I don’t think anybody then could have imagined where we are today. I was trained as a biochemist when neuroscience wasn’t even really a field. Back then it was called bio-psychology. Then, when I started graduate school, there was only one neuroscience department in the country: the Harvard neurobiology department. A few years later, Johns Hopkins opened the second department.
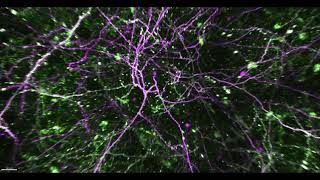
Then, over the last 15 or 20 years, there has been a revolution of new, critical techniques. For example, we can now do human genome sequencing in a day, and the imaging techniques we now use are incredibly sensitive, high-resolution tools that can produce incredible visuals from inside the brain, while an animal is behaving.
We can control the activity of neurons in the brain, stimulating certain neuronal circuits to induce behaviors by using light. This ability to image and manipulate brain activity, we never could have imagined 30 years ago.
We can now image a million synapses at a time and watch them before and after learning. The problem is that we can see changes, but we don’t know exactly where the changes are happening. Investigators in the neuroscience department are collaborating with researchers in the biomedical engineering department to identify synapses in space and time, using artificial intelligence, machine learning and computational approaches. Ten years ago, that was unfathomable.