Headed for his fourth liver resection, Bob Charnley had had enough. Surgery was almost as bad as the cancer itself. Homebound and desperate, he did what doctors advise patients not to do: Charnley Googled.
And when he Googled, he came across the Johns Hopkins Division of Nuclear Medicine and Molecular Imaging, where he found that recent advances in positron emission tomography (PET), a decades-old technology undergoing a reinvention, might spare him additional surgery. That search proved a lifesaver for Charnley, as it has for a steadily growing number of patients with cancer who have benefited from so-called theranostics over the last few years.
“Theranostics is a subfield of nuclear medicine that uses powerful diagnostic imaging to spot tumors throughout the body, and it combines that capability with therapeutic approaches to target the tumors and destroy them,” says Martin Pomper, director of nuclear medicine and molecular imaging at Johns Hopkins. “We can use PET to see cancer and can kill it using the same biochemical principles, by merely changing the radionuclide from one for imaging to one for therapy.”
For PET, after years taking a back seat to other forms of medical imaging — such as CT and MRI — the diagnosis-and-cure-in-one promise of theranostics has put nuclear medicine in the driver’s seat of a new era of precision medicine, tailoring care to individual patients, like Charnley, in ways that were not possible only a few years ago.
“Nuclear medicine is no longer just an imaging technique. It’s a therapeutic tool as well,” Pomper says. “We can cure people with it.”
Postwar Promise
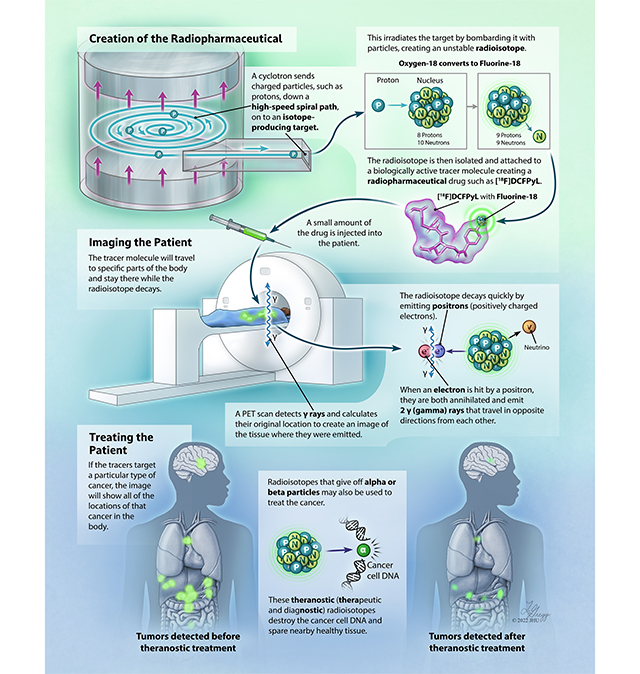
Nuclear medicine first took flight in the postwar period, capitalizing on the steady flow of radioactive isotopes emerging out of Manhattan Project-era atomic energy research and the growing availability of cyclotrons needed to manufacture isotopes.
In nuclear medicine, these radioactive isotopes are joined, or “tagged,” to synthetic compounds or biomolecules that are naturally — or designed to be — attracted to specific cells of interest in the body: neurons in the brain, heart muscle, lungs, liver and so forth. The targeting molecules can be drawn from a wide variety of biomolecules: antigens, peptides, enzymes, even sugars.
Trace amounts of these radioactive molecules are then injected into the patient’s bloodstream, traveling through the body and concentrating as “hot spots” in the target cells. As the radioactive molecules collect in the targeted tissues, they emit radiation that can be imaged or otherwise recorded, providing a detailed look inside the body.
Johns Hopkins’ experience with nuclear medicine dates to the late 1950s and the arrival of Baltimore-born Henry Wagner ’52, who became known worldwide as the father of modern nuclear medicine. After completing his internship and residency at Johns Hopkins, Wagner joined the Department of Radiology in 1957 and later founded the Division of Radiation Health Sciences in the school of public health. His earliest experiments looked at the diagnosis of pulmonary embolisms and brain neuroreceptors.
Over his long career, Wagner led in the lab, at the bedside and in the classroom. He cared for patients, mentored hundreds of future specialists in nuclear medicine, authored numerous peer-reviewed studies and, when necessary, volunteered himself as guinea pig in his own studies. When Wagner wanted to study a tissue, often it was his tissue that got imaged.
“Henry Wagner was the first dopamine-receptor PET subject in 1983 and, later, when we made carbon-11 carfentanil to study opiate receptors, he was the first subject in that study too,” says Robert Dannals, Ph.D. ’81, who directs PET radiochemistry at Johns Hopkins and was one of Wagner’s mentees.
The Power of PET
While the first positron imaging device (detector) was developed in the 1950s, it was not until the 1970s when groups of these detectors were working harmoniously to produce the early PET images that are recognized today.
For the field’s first few decades, new isotopes were the rallying cry. Companies sprang up to produce myriad isotopes — indium-111, technetium-99m, gold-198 and so on. The first true theranostic was radioactive iodine, used to image the thyroid and then treat thyroid disease.
The best-known and most widely used PET isotope today is fluorine-18, which was most effectively combined with a sugar analog to create fluorodeoxyglucose, better known as FDG, in the 1970s. FDG was a true breakthrough. Solid tumors in the liver, lungs, pancreas and elsewhere crave energy to grow. FDG gets metabolized more rapidly by the tumors than in surrounding healthy tissues, concentrating the isotope in the tumors.
The same metabolic principle is applicable throughout the body. In cardiology, for instance, the inability of dead or damaged heart muscle to metabolize FDG creates a contrast between damaged and healthy tissues to help diagnose heart damage. With these nuclear techniques, cardiologists at Johns Hopkins can noninvasively examine blood flow in the heart muscle, diagnose dysfunction in the heart’s contractions and gauge metabolism in heart tissue to detect conditions ranging from coronary artery disease to cardiac sarcoidosis.
The introduction of FDG stimulated the development of PET scanners. As they decay, a certain subset of isotopes emits atomic particles known as positrons — positively charged electrons. Whenever a positron and an electron collide, the two annihilate each other, yielding two photons that rocket away from each other in diametric opposition — exactly 180 degrees apart. This tidbit of Manhattan Project-era physics is the cornerstone of PET imaging.
PET scanners are typically designed in a ringlike or tube shape with sensors surrounding the entire body. When sensors on opposite sides of the body detect two photons, a mathematical line can be calculated between the two positions, tracing a direct path back through the body to the precise spot where the photons originated. In other words, they denote the exact location where the radioisotope is within the body. And many such lines, considered together, generate a picture of the nearby tissue.
A Rapidly Evolving Field
PET’s diagnostic capabilities are only one-half of the theranostics equation. The more recent and perhaps most promising advances are on the therapeutic side. Using the same biochemical targeting capabilities but attaching isotopes whose radioactivity manifests differently, physicians can kill the same cancer cells they just located. It was theranostics that arrested the growth of the tumor that held Charnley in its grip.
“Bob was fortunate. His tumor was actually a metastatic form of a neuroendocrine tumor with a well-known biomarker, and we were able to target it with a medicinal theranostic agent,” says Lilja Solnes, a Johns Hopkins nuclear radiologist who helped treat Charnley’s cancer by targeting somatostatin receptors in his tumors. “Theranostics not only extended Bob’s life; it improved his quality of life too.”
Solnes is now working with colleagues in the PET Center to study several promising biomarkers and pairing them with radioisotopes. Many of the biomarkers have long been known but are only now being translated into patient care.
“The field is evolving rapidly,” she says. “There are many more agents coming soon. We have some in the pipeline that will be a paradigm shift in imaging and treating cancer.”
Instead of positrons, the isotopes used in therapeutic nuclear medicine give off alpha and beta particles. An alpha particle, 7,000 times heavier than its beta counterpart, cuts through cancer DNA like a razor, killing the tumor cells one by one.
The Half-Life Sciences
The key in both diagnostic and therapeutic nuclear medicine is to find isotopes that last long enough to make it to the tissue in question and remain there long enough to do their jobs, then decay away quickly. When carefully timed and precisely targeted, these therapeutic isotopes destroy the cancer cells and spare nearby healthy tissue to the greatest degree possible.
The rate at which an isotope decays is known as its half-life — the length of time it takes a given amount of radioactive material to decay by half. Eventually, the isotope decays to nothing and disappears from the body.
“The half-lives of isotopes are very precise,” says Dannals, a radiochemist who has spent much of his career chasing new isotopes with varying half-lives. “You try to choose the isotope with a half-life that’s suitable for the biology you want to examine — or to kill.”
The class of radioisotopes that emit positrons have half-lives ranging from about two minutes to a little under two hours. The fluorine-18 used in FDG has a half-life of precisely 109.7 minutes. By comparison, carbon-14, a familiar isotope used to date ancient artifacts, has a half-life of some 5,730 years. The short half-lives of many isotopes used in nuclear medicine demand a careful orchestration to both produce the radiotracer in quantity and then quickly get it into the patient. These isotopes cannot be ordered from afar; they must be made on-site.
“Carbon-11 has a 20-minute half-life,” Dannals says, “After about an hour — or three half-lives — your images are already starting to get fuzzy.”
Dannals notes that the decadeslong search for new isotopes is now more or less mature. There are seemingly few isotopes left to discover. Driven by the promise of theranostics, however, the race is now on to find new biochemical tracers — the next FDG — that can be used to target new tissues and tumors in the body.
New Standard of Care
Until just the last few years, Pomper says, FDG was used in 95% of all PET imaging, but that dynamic has started to change. In 2021, after a quarter-century of research, Pomper himself made international headlines with the introduction of a small-molecule PET agent targeting the prostate-specific membrane antigen (PSMA), an enzyme found on the surface of prostate cancer cells. He has used it to create a theranostic approach that both identifies prostate cancer even when it has metastasized in other parts of the body and paired it with a therapeutic agent that destroys the cancers.
The technology’s specificity and resolution can detect lesions as small as a few millimeters wide, leading to earlier detection. PET is ideal for monitoring of treatment efficacy as well.
“You can watch as the tumors shrink,” Pomper says. More intriguingly, PSMA is also found in the nascent blood vessels of all solid tumors. Pomper believes the biochemistry involved might help detect other cancers, like those of the kidney and brain, known to have high levels of PSMA.
PSMA is turning heads worldwide. Pomper recently won the Gold Medal from the World Molecular Imaging Society for his discovery of small molecules targeting PSMA.
“It is one of the greatest science-to-practice innovations in medical history,” says Pamela Johnson, vice president of care transformation for the Johns Hopkins Health System and Pomper’s colleague in radiology. “Shortly after FDA approval, PSMA imaging became a recommended diagnostic test in the National Comprehensive Cancer Network guidelines. Patients around the world are now benefiting from this new and very promising standard of care.”
“The most interesting question that we’re working on now is understanding when and how the brain is healing or, in the case of so many former football players, why it is not.”
—Jennifer Coughlin
The Mind’s Eye
Another area of research that has proved fruitful for nuclear imaging is the brain. Jennifer Coughlin is a Johns Hopkins psychiatrist who is using nuclear imaging to study a broad range of brain functions and dysfunctions. She’s studying tennis players and swimmers, patients with HIV, and those with memory disorders, looking at everything from how the healthy brain ages to schizophrenia and depression, even traumatic brain injury.
In one study, Coughlin is using a biomarker of neuroinflammation to study brain injury and repair in former National Football League players. PET is helping her to look for indications of inflammation from past brain injury, which is implicated in chronic traumatic encephalopathy (CTE), a serious condition common among former players that is tied to repeated head injuries. Right now, CTE can only be diagnosed postmortem. Coughlin’s approach is allowing her to peer into the brains of living former players for signs of inflammation, damage and, she hopes, healing.
“The most interesting question that we’re working on now is understanding when and how the brain is healing or, in the case of so many former football players, why it is not,” Coughlin says of the insights she’s gaining with PET. “We’re tracking players where it seems like the injury is persisting, even after they step away from the game, to understand what makes their brains vulnerable.”
On her webpage, Coughlin posts an open call for test subjects to participate in a long and varied list of her current studies, many in collaboration with Pomper. In one study, Coughlin is using PET to build on existing research that shows people with active major depressive disorder have notably high levels of translocator protein (TSPO), a biochemical that plays a role in the activation of the brain’s immune cells, known as the microglia, causing inflammation. The thinking is people with this inflammatory subtype of depression may benefit from a dual treatment with antidepressants and anti-inflammatory medications.
“There are limitations to TSPO as a biomarker,” Coughlin cautions. “We have now identified a more specific biomarker and can use PET to study this inflammation and, hopefully, discover some first-ever ways to improve microglial activation in patients with treatment-resistant depressions.”
Coughlin is similarly proud of her clinical work with young adults with mood disorders. She is hopeful that PET might give her the ability to someday visualize mental illness — which could be very valuable for her patients.
“Many young adults struggle to accept any disease or diagnosis,” Coughlin says of how she plans to use PET scans in her work. “Having an image showing that this is not their fault, that there really is a biomedical change in their brain that needs treatment, will be an invaluable tool in that work.”
Precision Health
PET imaging and theranostics remain relatively expensive. A single scan can cost thousands of dollars. With such clear value proposition for PSMA and other theranostics, however, Pomper thinks inexpensive screening will become an important part of precision health and value-based patient care, helping to identify patients at greatest risk and the greatest need for these new techniques.
“I can imagine using liquid biopsies — an inexpensive blood screening test — to look for tumor DNA in the blood and help us identify patients who will benefit most,” Pomper says. It takes a tumor of perhaps 50 million cells for DNA to be detectable in the blood. “If you get a hit, you can then order up the scan and see all tumors with a similar number of cells.”
All in all, the growing attention being paid to nuclear medicine, and PET with theranostics in particular, has put Pomper and his colleagues in a relatively unfamiliar place: in front of patients. Pomper foresees a new era for radiology in which these once behind-the-scenes physicians step out from behind the scanners to take an active role advising and guiding patient care.
“These promising new agents are the products of our field, and we are delighted to see their expanding use to benefit our patients,” Pomper says. “This moment is a remarkable opportunity for nuclear medicine to step to the fore and lead in patient care.”




