Celebrate our 50th Anniversary by exploring our dedicated website for a treasure trove of memories and exclusive anniversary podcasts. Dive in now to be part of our
special celebration.
-
Cancers We Treat
At the Johns Hopkins Kimmel Cancer Center, our experts are dedicated to providing our patients the best treatment and quality of care possible. Patients are seen by multi-disciplinary teams for individualized treatment plans.
-
Clinical Trials
At the Kimmel Cancer Center, our experts recognize that cancer is a complex disease, and each patient is unique.
-
Our Experts
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins includes a wide array of medical professionals and laboratory scientists. Many of our experts are nationally and internationally recognized as leaders in the research and treatment of cancer.
-
Kimmel Cancer Center in Washington DC Region
The Johns Hopkins Proton Therapy Center in Washington, D.C., is one of the largest and most advanced centers in the U.S. Learn more about cancer services in Suburban Hospital and Sibley Memorial.
-
News
Stay up to date with the latest developments in research and treatments from our experts. Read our current news releases and subscribe to our blog, Podcasts, YouTube channel and follow us on Facebook, Instagram and Twitter.
-
Patient and Family Services
The Harry J. Duffey Family Patient and Family Services Program offers a variety of resources to assist during this time, such as emotional support, assistance with temporary housing and transportation and aid in managing care.
Request An Appointment
Before Making the Call
410-955 8964
The Johns Hopkins Hospital
1-855-695-4872
Outside of Maryland (toll free)
+1-443-219-6052
International
-
Community Outreach and Engagement
At Johns Hopkins, we recognize our obligation to serve and educate the community and to ensure that new discoveries and knowledge are disseminated at the community level.

-
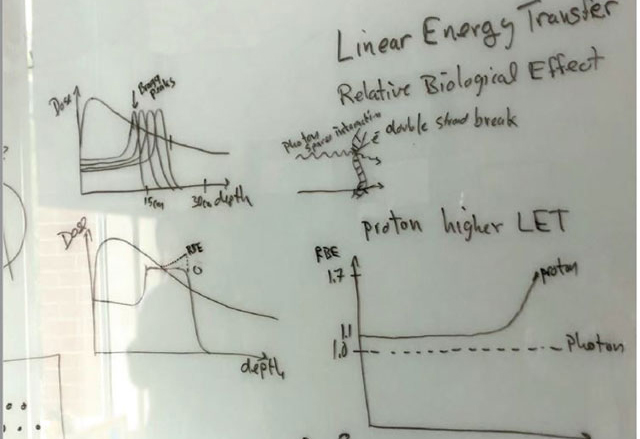
Our Research
How we conduct our research and translate those results aims directly at the core of our mission: Improved patient care.
-
Our Locations
Our centers are available in both the Baltimore and Washington, DC metro areas.
Publications
-
Breast Matters
A publication by the Breast Cancer Program at the Johns Hopkins Kimmel Cancer Center.

-
Promise & Progress
The magazine of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.

-
Kimmel in the Community
The Johns Hopkins Kimmel Cancer Center is in your community.

