-
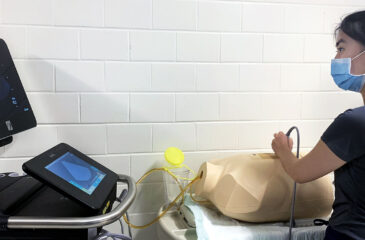
9th Annual Advanced Pediatric Anesthesiology Fellowship Bootcamp
The Department of Anesthesiology and Critical Care Medicine at the Johns Hopkins School of Medicine welcomes your pediatric anesthesia fellows for a one-day simulation training event. For the last nine […]
-
Johns Hopkins Anesthesiology Program Ranked #1
We are thrilled to report that Johns Hopkins University School of Medicine Anesthesiology was ranked #1 and is one of seven specialty programs ranked among the top five by U.S. […]
-
AI Model Predicts Delirium in ICU Patients
Hopkins Medicine neurointensivist Robert Stevens and Hopkins Engineer students and faculty collaborated to develop an #AI model that predicted delirium in ICU patients. More than one-third of all people admitted […]
In The News
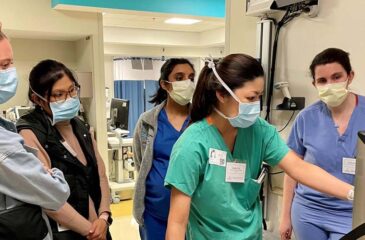
The Sadie Abell Senior Pediatric Critical Care Medicine Bootcamp 2024
The Department of Anesthesiology and Critical Care Medicine at the Johns Hopkins School of Medicine welcomes your pediatric anesthesia fellows for a three-day simulation training event at the Johns Hopkins […]
Education
9th Annual Advanced Pediatric Anesthesiology Fellowship Bootcamp
The Department of Anesthesiology and Critical Care Medicine at the Johns Hopkins School of Medicine welcomes your pediatric anesthesia fellows for a one-day simulation training event. For the last nine […]
Research
ACCM Cardiac Anesthesia SCA Presentation Guide
The 46th SCA Annual Meeting and Workshops will be held April 27-30 in Toronto, Canada. Once again, Hopkins Cardiac Anesthesiology members are making meaningful and important contributions to the meeting […]
Departmental Accolades
Dr. Fernandez Receives Women’s History Month Achievers Award
Allison Fernandez is passionate about women’s empowerment in medicine. As a leader in the pediatric anesthesiology arena, Fernandez works to ensure women and girls have the resources they need to […]
Grand Rounds
Grand Rounds Webcast, April 18th
Watch “Drug Development For Pulmonary Arterial Hypertension: From Phosphodiesterase-5 To Pyruvate Dehydrogenase Kinase And Dynamin Related Protein 1” with Visiting Professor Stephen Archer from Thursday, April 18th’s Grand Rounds. Speaker: […]
ACCM Achievements
ACCM Achievements
Brandon Sumida, MD served as the ACCM lead/host for multiple Hopkins events for Pathways in Technology Early College High School (P-Tech) students from Paul Laurence Dunbar High School. In December, […]
Search
We are celebrating our and all Doctors!
Thank you for all you do for our patients.
Join us as we celebrate Tech Week!
Our incredible Coding & Collections team won a Best Team Happy Hour celebration at Lib’s. They really are the best – grateful for their expertise and teamwork!
A great presence for Johns Hopkins at American Academy of Pain Medicine.
Discovery and Innovation Calendar
View this month's calendar