Published in
Discovery -
Winter 2021
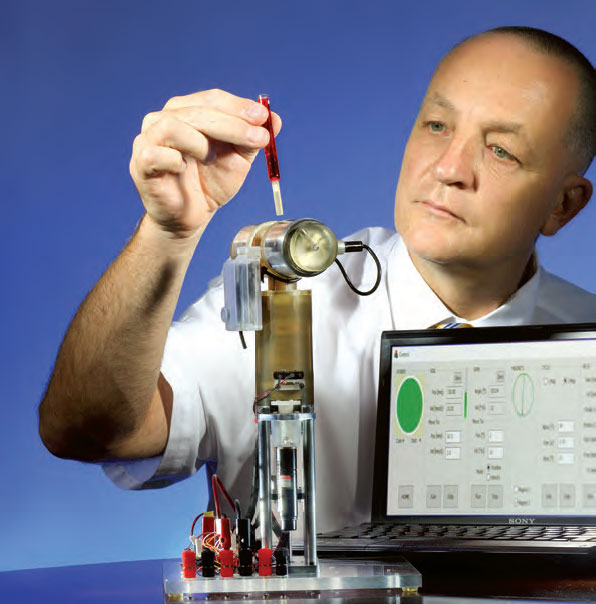
The Brady’s own Dan Stoianovici, Ph.D., Director of the Urology Robotics Program and inventor of numerous robotic devices, has done it again: With the help of surgeon Misop Han, M.D., the David Hall McConnell Professor in Urology, Stoianovici has developed a novel robotic ultrasound probe especially for the prostate. The work was supported by the Patrick C. Walsh Prostate Cancer Research Fund.
“The probe integrates ultrasound and robotic components within the same structure, and it is the first construction of this kind,” Stoianovici says. Its anticipated uses are not only for better imaging of the prostate, but also for “image-guided interventions,” says Han: “Currently, ultrasound probes are mostly operated manually. Robot assistance will allow hands-free probe operation, 3-Dimensional ultrasound scanning, and automated needle targeting for ultrasound-guided procedures such as biopsy and ablation.”
Stoianovici’s lab has built a prototype of the new probe, and used it in preclinical tests – that were so promising, the FDA has approved the device for clinical trials.
Today’s targeted biopsy fuses the results of prostate multiparametric (mp) MRI with ultrasound. Although this combined imaging approach is much more accurate than the traditional transrectal ultrasound biopsy, there is still room for improvement. “Targeted biopsy misses a significant number of clinically significant prostate cancers,” says Stoianovici, “because of errors in fusion between the MRI and ultrasound, and in manual targeting. In addition, not all clinically significant prostate cancer is detected in MRI.” Another drawback, notes Han, is that “the lesions detected by mpMRI that we sample in targeted biopsy are not necessarily those that are biologically dangerous. Further advances in technology are needed, and the new probe has the potential to make our targeted biopsy even more accurate.”
Stoianovici’s lab has built a prototype of the new probe, and used it in preclinical tests – that were so promising, the FDA has approved the device for clinical trials. “Based on this approval, the Johns Hopkins University IRB has recently approved the study protocol, and we plan to start a safety and feasibility trial soon.” Ultimately, Stoianovici believes, the robotic probe has the potential to “reduce the dependency of clinical results on physician’s skill and training, and also reduce variability among physicians.”