For Faculty and Sponsors

Range of Services
The Drug Development Unit in collaboration with the Clinical Pharmacology Analytical Laboratory (CPAL) provides clinical trial service support to Johns Hopkins investigators and pharmaceutical, federal and non-profit sponsors. Investigators and sponsors can choose among a range of services, including:
- Development of clinical research protocols from an initial concept
- Preparation of IRB and Clinical Research Unit submissions
- Clinical study implementation and conduct by highly trained research staff
- Laboratory sample processing, storage, and shipment
- Drug assay development and analysis in various fluids and tissues
- In vitro simulation of in vivo pharmacokinetic processes
- On-site quality assurance by a research nurse manager
- Pharmacokinetic and pharmacodynamic data analysis
- Manuscript preparation
For information about conducting research with the DDU, contact:
- DDU Associate Director Phone: (410) 614-8762
- DDU Fax: (410) 955-9708
DDU Experience

Throughout its history, the DDU has been committed to supporting and collaborating on a diverse range of study designs, including studies with special populations, with investigators throughout the Johns Hopkins Medical Institutions. Similarly, the DDU has wide-ranging experience in collaborating with commercial and federal sponsors.
Study Designs
- First-in-Human
- Pharmacokinetics (Phase 1)
- Pharmacodynamics (Phase 2)
- Drug-Drug Interaction
- Methods Development
- Limited Phase 3
Special Populations
- Healthy Volunteers
- HIV, HCV, TB
- Intensive Care
- Pregnancy
- Lactating Women
- Pediatrics
- Healthy Gerontology
- Dermatologic Disease
- Rheumatologic Disease
- Hepatic Impairment
School of Medicine Collaborations
- Infections Disease
- Gastroenterology
- Pulmonary
- Rheumatology
- Endocrinology
- Dermatology
- Gynecology & Obstetrics
- Neurology
- Ophthalmology
- Pathology
- Pharmacology and Molecular Science
- Psychiatry
- Pediatrics
- Radiology
- Surgery
Sponsors
- 30 Industry Sponsors
- National Institutes of Health
- Centers for Disease Control
- Food and Drug Administration
- Department of Defense
- U.S. Agency for International Development
Services
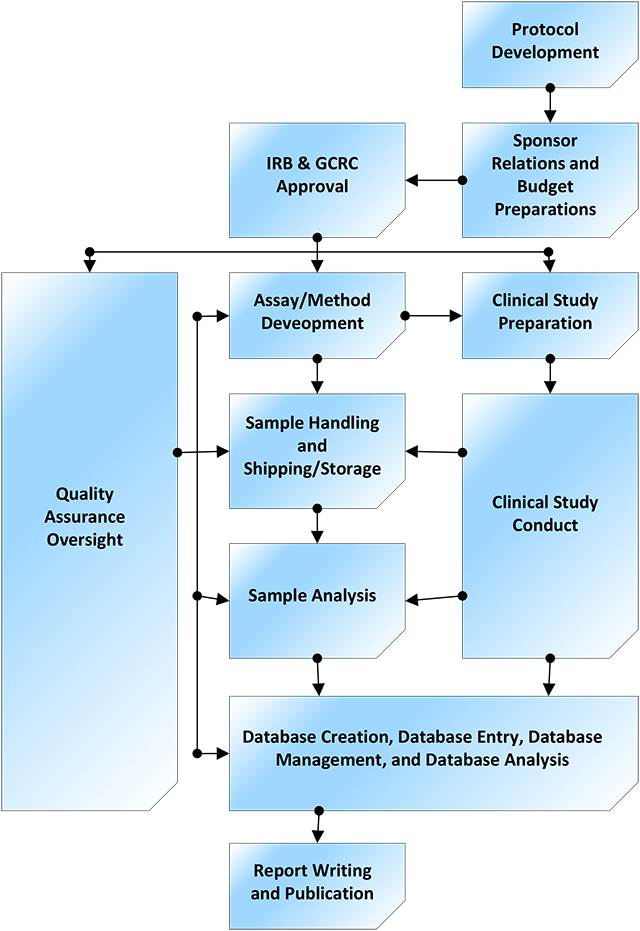 Investigators and sponsors may select desired services from anywhere along the clinical development pathway, from initial concept to publication. View PDF.
Investigators and sponsors may select desired services from anywhere along the clinical development pathway, from initial concept to publication. View PDF.The Drug Development Unit in collaboration with the Clinical Pharmacology Analytical Laboratory offers a full range of services to Johns Hopkins faculty and sponsors in support of conducting clinical research.
- Full service: concept to publication
- Investigator/Sponsor selects services and expertise needed
- DDU Faculty provide oversight throughout the study
- Faculty/fellow/nursing training available
