Local Drug Delivery to Brain Tumors
History
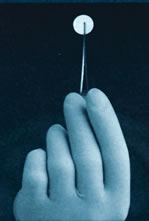
Gliadel®, a biodegradable polymer
loaded with the chemotherapeutic
agent called BCNU
In our Hunterian Neurosurgical Research Laboratory, our team seeks out ways to develop local drug delivery techniques that allow direct access to tumors while avoiding the adverse effects of standard systemic drug therapy. Under the direction of Dr. Henry Brem, the strategies of local drug delivery devised in the lab have had immediate impact on the care of patients with brain tumors.
In fact, in 1995 the FDA approved the use of Gliadel®, a biodegradable polymer loaded with the chemotherapeutic agent BCNU, for the treatment of recurrent gliomas. It was the first product newly approved by the FDA for the treatment of malignant brain tumors in over 23 years.
The laboratory experiments which led to clinical trials with GLIADEL®, and ultimately to its approval for commercial use, were all performed in the Hunterian Laboratory.
Researchers at the Johns Hopkins Comprehensive Brain Tumor Center also developed the GliaSite Radiation Therapy System, which delivers radiation from within the hole created by the surgical removal of a malignant brain tumor.
Where We Are Now
In addition to polymer-based delivery systems, Johns Hopkins, in cooperation with the Massachusetts Institute of Technology, have been exploring alternative devices to locally deliver multiple drugs in precisely timed regimens.
One of our recent developments is a novel microelectromechanical device (microchip) for local drug delivery. This device represents an alternative to our established polymer-based systems and offers the advantage of extremely precise control of drug delivery. Chemotherapeutic agents released from this device demonstrated growth inhibition of implanted rodent tumors. BCNU delivered from the devices was as effective as subcutaneous injections of BCNU at inhibiting tumor growth.
Future Directions
Since the biological activity of treatments largely depends on obtaining sufficient concentrations at targeted sites and penetration of the blood-brain barrier requires prohibitively large systemic doses, new agents will be incorporated into controlled local delivery systems and evaluated to establish their safety and efficacy against intracranial models of malignant gliomas.
The laboratory will be investigating the efficacy of locally delivered agents, including RNA interference (RNAi) sequences, cytokine-retargeted adenoviruses, inhibitors of glutamate-mediated invasion, and anticancer ribonucleases (RNAase).
We will also be investigating the effectiveness of chemotherapy in combination with various agents which have differing and complementary mechanisms of action, such as inhibitors of glutamate-mediated invasion and inhibitors of angiogenesis, resistance-modifiers, immune stimulators, and chemotherapy in combination with a variety of biological agents that intervene in critical pathways of tumorigenesis and tumor growth.
We also plan to continue to build on the laboratory’s successful investigational approach to include technologically advanced drug delivery systems delivering novel and effective chemotherapeutic and biological agents that can best be of benefit to patients if delivered directly to the brain. We will be investigating various polymer types, composition, and geometric configuration, to allow sustained delivery of more effective drug concentrations.
A variety of new configurations such as sheets, rods, microspheres, and nanospheres will also be tested and may permit much broader clinical applications and less invasive implantation techniques, such as through stereotactic or endovascular injection.
