The 10-year-old girl was dying.
It was 1984, and Nicole was the first patient at The Johns Hopkins Hospital to receive a bone marrow transplant from an unrelated donor. She had been injected with healthy marrow, rich in disease-fighting immune cells, to replace material that had been decimated by cancer and radiation. But the donated immune cells were attacking her organs. The condition, known as graft-versus-host disease, occurs in about half of bone marrow recipients and is now treated with drugs that suppress the immune system. These days, it is rarely severe enough to be fatal.
At the time, Drew Pardoll was an oncology fellow, saddled with the searing assignment of caring for a child who would not live to adolescence. One night, as he flipped through the science journal Nature, an article about the workings of the immune system caught his attention. He thought about Nicole’s foreign immune cells, attacking both cancer tumors and healthy organs, powerful enough to kill her. And he contrasted her experience to that of bone marrow transplant patients who survived graft-versus-host disease, their cancer beaten back by the same immune system attack.
“I decided two things,” says Pardoll, 58, leaning forward in a leather chair in his fourth-floor corner office of the Bunting Family and Jacob and Hilda Blaustein Family Cancer Research Building. “One was that the immune system was the most powerful anticancer weapon that we had, more powerful than any drug. The other was that we had to understand how to regulate it so we could focus it more on the cancer.”
With that “aha” moment, Pardoll began decades of research, at first in obscurity and now as a leader in immuno-oncology, a field that is transforming cancer treatments by tapping the power of the immune system to shrink and eliminate tumors. Today, FDA-approved treatments and clinical trials based in part on Pardoll’s discoveries are extending the lives of people with melanoma, lung cancer and other forms of cancer.
“Drew and his team are leading research that not only tremendously advances the field, but also generates new optimism about cancer treatments,” says Landon King, executive vice dean of the Johns Hopkins University School of Medicine.
Turning immune system insights into life- saving drugs, however, is a long and expensive process, requiring substantial investment and no promise of success. “The big cost jumps are when you go into clinical trials,” says Pardoll, director of the Cancer Immunology Program. “The funding system in academic centers can’t support that.”
To move his ideas forward, he teamed with investors to start an immuno-oncology com- pany, Amplimmune, in 2006. When it was acquired by MedImmune for $500 million in 2013, investors took notice. Last year, Pardoll joined other immuno-oncology entrepreneurs to create Potenza Therapeutics in Cambridge, Massachusetts.
These business deals are lucrative for the researcher and Johns Hopkins, and they provide funding and business support critical to shepherding his discoveries forward. Says Pardoll: “The only things that matter for me are FDA approval and having the therapies available around the world.”
Immuno-Oncology
The human immune system is astonishingly complex and nimble, one day fighting the flu and the next making sure a paper cut stays infection-free. “It has to be ready for any invader,” says Pardoll. “Chemotherapy is one drug at a time. The immune system is trillions of different drugs.”
The idea of tapping this powerful internal weapon is not new. More than 120 years ago, William Coley, a bone surgeon at New York’s Memorial Hospital (now the Memorial Sloan Kettering Cancer Center), noticed that patients with cancer who got infections often saw their tumors shrink or disappear. In 1891, Coley began testing the phenomenon by injecting his patients with bacteria to spark an immune system response against the cancer.
Despite some success—and many excruciating failures—the procedure fell out of favor, particularly after radiation became the standard cancer treatment at the turn of the 20th century.
Coley’s theory was not in dispute. Scientists just had to figure out why immune systems are so good at vanquishing so many invaders yet so bad at stopping the spread of cancer. And they had to figure out how to tell immune system cells to attack cancer but spare healthy parts of the body.
Pardoll began tackling those questions when he was 27. Under the guidance of Johns Hopkins professors Donald Coffey and Bert Vogelstein, his Ph.D. advisers, he studied the immune system at the National Institute of Allergy and Infectious Diseases, a place devoted to research of immune response disorders.
“Don, Drew and I had weekly meetings about the best way to eventually conquer cancer, and [studying the immune system] was one of the approaches we discussed often,” says Vogelstein, whose own pioneering work has focused on the genetic mutations that cause cancer.
Preparing to Attack
ANIMAGRAFFS/ Jacob O’Neal
Pardoll was used to taking the lead. He had skipped three grades while growing up in Elizabeth, New Jersey, becoming a Johns Hopkins undergraduate at age 15. He enrolled in the school of medicine just three years later. But this quest could not be rushed. When Pardoll joined the Johns Hopkins faculty in 1988, he was still far from his goal of developing immune-based cancer vaccines.
“You have to take 10 shots on goal to score that one goal,” he says. “Cancer has beaten us on a lot more occasions than we’ve beaten cancer.”
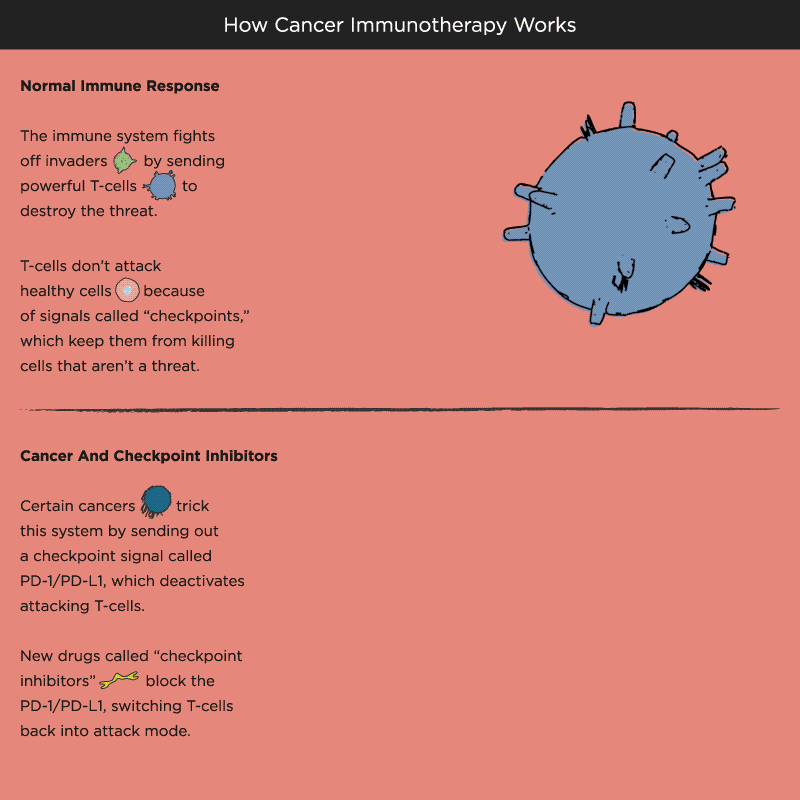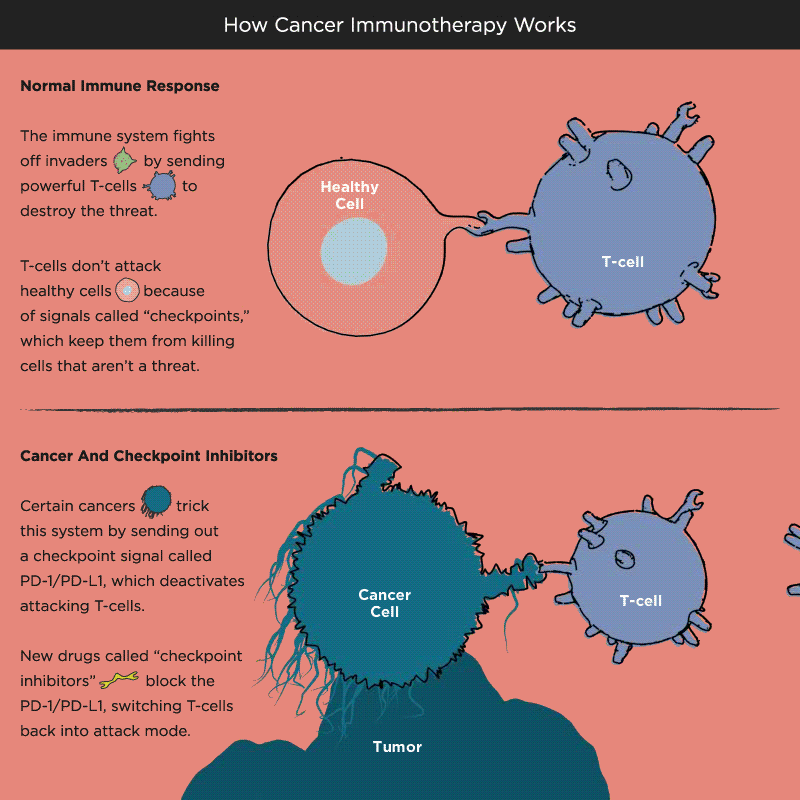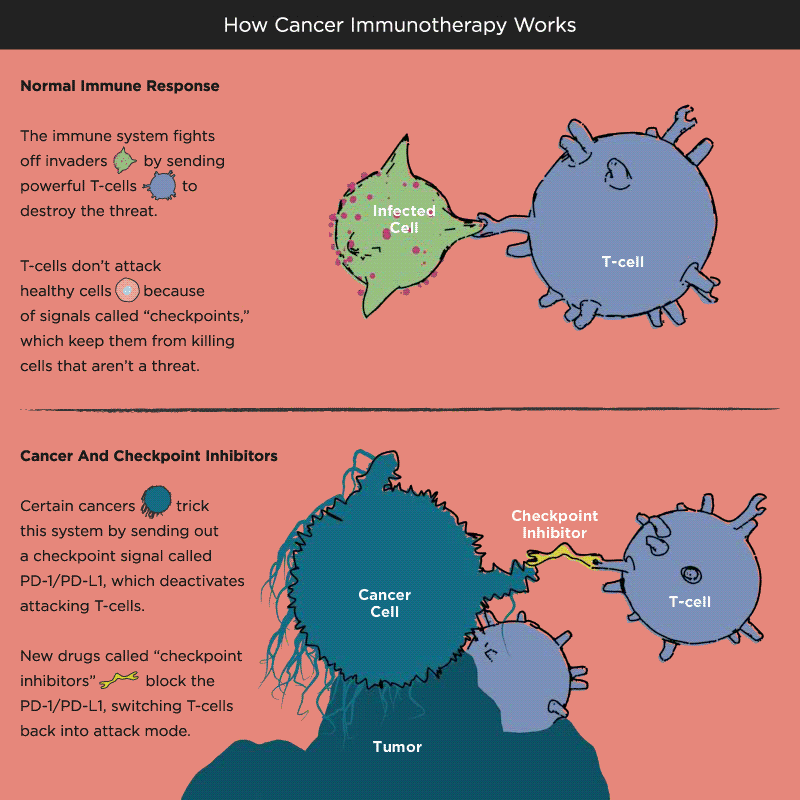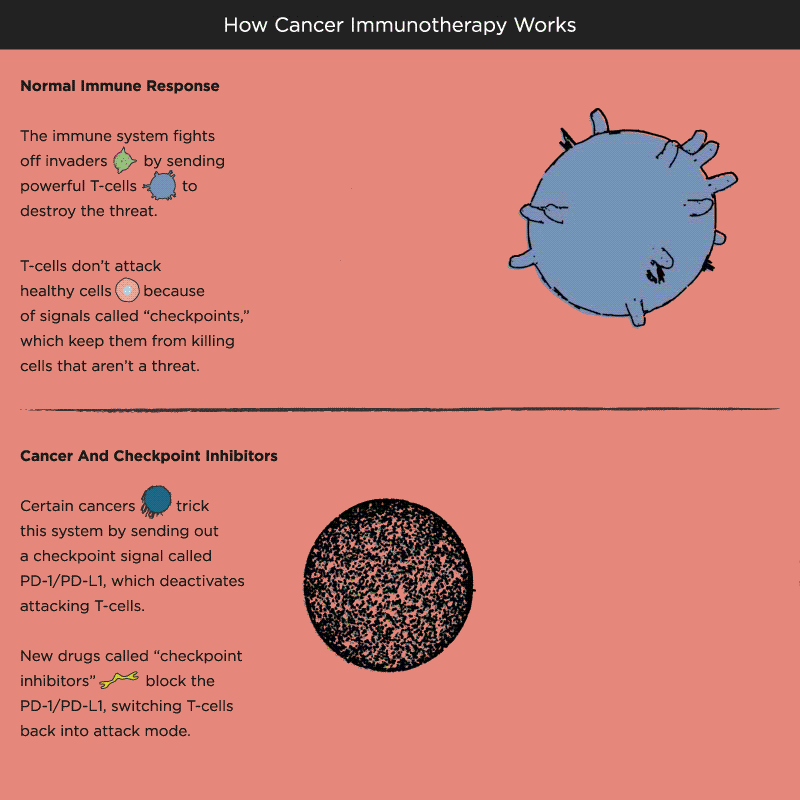
Over time, Pardoll and other researchers began to understand how cancer cells protect themselves from the immune system and its T cells. Think of T cells as the troops in a hardworking, multitasking army, working to keep the body healthy by recognizing and attacking invaders. When the battle is victorious and good health has been restored, a protein receptor on the T cell, known as a checkpoint, tells the troops to stop fighting, like an army sergeant delivering a “stand down” command. But cancer cells also emit signals telling T cells not to attack. As a result, the army backs down, allowing tumors to grow and spread.
Immuno-oncology research at leading academic medical centers around the world focuses on creating “check-point inhibitors” that block these “don’t attack” messages, freeing the T cells to vanquish cancer. Much of that work centers on an immune system brake expressed by T cells called PD-1. PD stands for programmed death, because T cells emit it when their work is done, as a way of calling off their immune system attack. PD-1 merges with molecules expressed by tumor cells to create a checkpoint brick wall that prevents the T cell troops from reaching the enemy cancer.
Today, Pardoll leads the Cancer Immunology Program at Johns Hopkins, where about 30 scientists are seeing dramatic progress in their pursuit of immunooncology treatments. “We expect the program to grow significantly with the many opportunities ahead,” says William Nelson, director of the Johns Hopkins Kimmel Cancer Center.
Starting a Company
These are klieg-light days for immuno-oncology. But back in 2006, the spotlight on researchers like Pardoll was considerably dimmer.
That’s when Arnold Oronsky came to Johns Hopkins in search of investment ideas. Oronsky, with a Ph.D. in immunology from Columbia University’s College of Physicians and Surgeons, is a partner of InterWest, a Menlo Park, California, firm that invests in early-stage health care and information technology. Pardoll and his team told Oronsky they had discovered several molecules expressed by cancer cells that interacted with PD-1 to block cancer cells from immune attack. Oronsky liked what he heard. “Because he had a background in asthma and allergy, he believed in the immune system,” says Pardoll. “We were not specifically thinking about creating a company until he proposed it.”
Pardoll came up with the name Amplimmune, because the company would develop therapies that amplify the potency of the immune system. Oronsky secured $10 million each from InterWest and from the Wellcome Trust, a U.K.-based foundation. In early 2007, the company hired its CEO: Michael Richman, a businessman-biologist who had run other cancer therapy companies.
“Amplimmune had exciting technology and prominent scientists, but they had a hole with respect to the business,” recalls Richman. “It looked like a great opportunity.”
The company launched in 1,500 square feet of laboratory and office space in the David H. Koch Cancer Research Building and moved to Rockville six months later. It now occupies a 20,000-square-foot facility in Gaithersburg, with on-site biologics manufacturing.
In August 2013, seven years after that initial $20 million investment, Amplimmune was purchased by its neighbor MedImmune, an arm of the global biopharmaceutical firm AstraZeneca, for $225 million, plus $275 million as agreed-upon milestones are reached.
“It made a lot of sense to bring Amplimmune into the MedImmune family,” explains Richman. “MedImmune had its own pipeline of immune therapy.” With Pardoll serving on MedImmune’s science advisory board, the company continues to develop therapies that “target the critical areas of the immune system that cancer can hijack to escape destruction,” says Ronald Herbst, MedImmune’s vice president for oncology research and development.
Soon after the acquisition, MedImmune and Johns Hopkins forged a five-year, $6.5 million research partnership, with both sides contributing funding and expertise to move forward several research projects, including immuno-oncology.
The sale of Amplimmune to MedImmune show-cased Pardoll’s business acumen as well as his research. “It made a big splash, with big numbers and high visibility,” says King. In the spring of 2014, MPM Capital asked Pardoll to be a founder of Potenza Therapeutics, an immuno-oncology startup.
Commercial Appeal
With Pardoll leading the way as co-chair of the Committee on the Innovation Ecosystem, launched in 2013, Johns Hopkins is helping researchers bring their ideas to the marketplace, either by licensing discoveries to companies or by forming enterprises of their own.
In 2015, the Technology Transfer Office was renamed Johns Hopkins Technology Ventures, and Christy Wyskiel, with a background as an institutional investor in medical technology and life sciences companies, was hired to helm it. A fast-track licensing program was rolled out, and a business incubation space, FastForward East, opened on Wolfe Street on the East Baltimore medical campus, a companion to FastForward Home- wood, which opened in the Stieff Silver Building in June 2013. Another innovation hub, at 1812 Ashland Ave., is scheduled to open in August 2016 with 25,000 square feet of space for startups.
Johns Hopkins researchers launch about 10 companies per year, says Wyskiel. “Yet few researchers are able to attract large institutional funding, as Pardoll did, and have a successful liquidity outcome.”
When commercialization pays off, as it did with Amplimmune, the inventors and their lab get half, and the rest is split between the school, department and university. In 2014, Johns Hopkins collected $16.5 million in licensing revenue, and leaders believe the potential for additional revenue is enormous.
Still, financial rewards are only part of the story. “Amplimmune is a success mainly because it brings cancer treatments that much closer to patients,” Wyskiel says.
“I’ve always been impressed with Drew’s approach to science,” says Vogelstein. “He tries to get at the basic scientific aspects first, understanding the biological aspects of the process. But he has always had therapy in mind, and as soon as he sees an opportunity for taking his benchwork to the bedside, he immediately implements it. This has meant more rapid translation of his ideas to the clinic than virtually any other researcher I know.”
As his therapies move forward in the private sector, Pardoll keeps learning more about the interactions between immune systems and cancer. He is investigating new checkpoint molecules and teaming again with Vogelstein, this time to study how cancers with different genetic compositions react to immune therapies. “This work brings together two fields of cancer research that normally do not interact,” he says.
There will be successes and failures, but Pardoll remains focused. “We don’t think there’s a single cancer that can ultimately beat the patient’s own immune system,” he says.
